1. For each of the following twelve species, draw an adequate
Lewis structure, including formal charges, give the
AXnEm class, predict the shape, and state if
the octet rule is being violated.
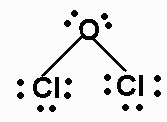
a) Cl2O
There are 20 valence electrons. The Lewis structure is:

The molecule is AX2E2, and is bent.
b) H2CO
There are 12 valence electrons. The Lewis structure is:

The molecule is AX3, and is triangular planar.
c) SF4
There are 34 valence electrons. The Lewis structure is:
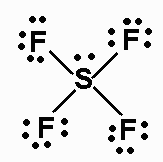
Note that the nbp on S is an equatorial nbp.
The molecule is AX4E, and has a seesaw shape (or distorted tetrahedron).
Note that a 2-dimensional Lewis structure does not show the shape. (The shape is inferred from
the VSEPR treatment.)
The octet rule is violated here.
d) CCl2Br2
There are 32 valence electrons.
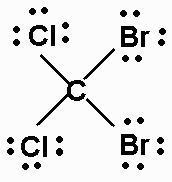
The molecule is AX4, and is tetrahedral.
e) ICl3
There are 28 valence electrons. The Lewis structure is:

The molecule is AX3E2, and is T-shaped (the 2 nbps are
equatorial).
The octet rule is violated here.
f) IF5
There are 42 valence electrons. The Lewis structure is:

The molecule is AX5E, and is square-based pyramidal.
The octet rule is violated here.
g) PF5
There are 40 valence electrons. The Lewis structure is:

The molecule is AX5, and is triangular bipyramidal.
The octet rule is violated here.
h) NO2-
There are 18 valence electrons. The Lewis structure is:

The ion is AX2E, and is bent.
We will soon see that there are two resonance structures for NO2-; this
is one of them; both N-O bonds are equivalent.
i) SO3
There are 24 valence electrons. The Lewis structure is:

The molecule is AX3, and is triangular planar.
The octet rule is violated here.
j) SO32-
There are 26 valence electrons. The Lewis structure is:

The molecule is AX3E, and is triangular pyramidal.
The octet rule is violated here.
There are three resonance structures for SO32-;this is one of them;all
three S-O bonds are equivalent.
k) OCS
There are 16 valence electrons. The Lewis structure is:
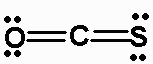
The molecule is AX2, and is linear.
l) PO43-
There are 32 valence electrons. The Lewis structure is:
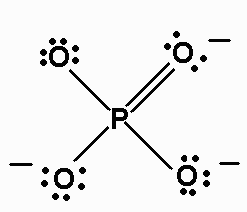
The molecule is AX4, and is tetrahedral.
The octet rule is violated here.
There are four resonance structures for PO43-;this is one of them;all
four P-O bonds are equivalent.
2. Match the molecules SiH4, PI3,
BBr3, GeCl2, and SF6 with the bond
angles 90o, 109.5o, 120o, slightly
less than 109.5o, and slightly less than
120o
.
SiH4, an 8 valence electron system, is AX4; it has 109.5o
bond angles.
PI3, a 26 valence electron system, is AX3E; its bond angles are
slightly less than 109.5o.
BBr3, a 24 valence electron system, is AX3; it has 120o
bond angles.
GeCl2, an 18 valence electron system, is AX2E; its bond angle is
slightly less than 120o.
SF6, a 48 valence electron system, is AX6;it has 90o bond
angles.
(Hint: for all of these species, count the valence electrons, deduce the
AXnEm class, and determine the geometry. With sufficient practice,
you can often do these steps without drawing a Lewis structure.)