
Need Book "Organic Notes" Purchase from Mrs. Marsden
Why study Organic Chemistry?
This is the chemistry generally practiced by living things. For biological efficiency, need readily available atoms and light atoms.
The chemistry is primarily based on C, H, N and O; also some P, Cl, Br, S (and some transition elements in enzymes)
At the moment ~ 107 organic compounds described in the literature either natural or unnatural
Why is carbon so great? Its multiple bonding structures allow a great deal of complexity from a few different elements.
Carbon can form stable C-X, C=X, CºX bonds, X = C, O, and N. No other element can do this. Thus, many different structural possibilities exist.
In the organic chemistry section, we are going to learn only 21 reactions - no new conceptual ideas.
Structures of Organic Compounds
Carbon is tetravalent - 4 covalent bonds. Carbon is usually positively charged

| Polarity | Bond | Bond Energy (kJ/mol) |
| very weak | dd -C-Hdd+ | 410 |
| C-C | 350 | |
| very strong | d+C-Od- | 350 |
| d+C-N d- | 300 | |
| polar | d+C-Cl d- | 335 |
| strongly polarized | d +H-Cl d- | 430 |
| d -N-H d+ | 400 | |
| Weak | O-O | 140 |
| I-I | 140 |
Nomenclature
Naming compounds - less important except for communication (it’s a pain, but less difficult than referring to "that thing with the thingy hanging off it."
Types of Compounds
| Bond Type | Class | E.g |
| CH, CC | Alkanes | H3C-CH3 |
| ethane | ||
| CH, CC, CN | Amine | H3CH2CNH2 |
| aminoethane | ||
| CH, CC, CO | Alcohol | H3CCH2OH |
| ethanol | ||
| CH, CC, CO | Ether | H3CCH2OCH2CH3 |
| ethyl ether | ||
| CH, CC, CX | Alkyl Halide | BrCH2CH2Br |
| X=Cl, Br, I | 1,2-dibromoethane | |
| C-C, C=C, CH | Alkene | H2C=CH2 |
| ethene | ||
| C-C, CH, C=O | Aldehyde | 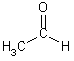 |
| acetaldehyde | ||
| C-C, CH, C=O | Ketone |  |
| (2-propanone) | ||
| C-C, CºC, CH | Alkyne | HCºCH |
| acetylene, ethyne | ||
| C=C, CH, | Aromatic | 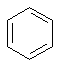 |
| benzene
|
||
| C=O, C-O, C-C, CH | Carboxylic Acid | 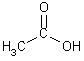 |
| acetic acid |
Alkane Nomenclature: Structural Isomers
| Number of Carbons | Root | Number of Isomers | ||
| 1 | Meth | 1 | ||
| 2 | Eth | 1 | ||
| 3 | Prop | 1 | 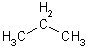 |
|
| 4 | But | 2 | 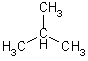 |
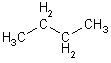 |
| 5 | Pent | 3 | ||
| 6 | Hex | 5 | ||
| 7 | Hept | 9 | ||
| - | ||||
| 10 | Dec | 75 | ||
| - | ||||
| 40 | 62,491,178,805,831 |
H3C - H ® H3C-CH2-H® H3C-CH2-CH2 - H
Take the previous analogue, replace H with CH2H to get higher homologue.
First, establish the
Bonding no double / triple bonds
e.g. CH4 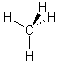
geometry for C (and all 2nd row elements)
4 atomic orbitals 1 x s (2s) spherically symmetric ![]()
3 x p 2 px x axis ![]()
2 py y axis ![]()
2 pz z axis 
2 rules i) Hunds rule - leave isoenergetic electrons unpaired
ii) maximize electrostatic repulsion (i.e., separate electronic pairs
as much as possible)
The carbon in CH4 has 4 bonds (one to each H). We need to use all four atomic orbitals to make the 4 molecular orbitals (4 SIGMA s orbitals). Mix 1 x 2s + 3 x 2p and get 4 x sp3 orbitals. In a tetrahedral compound, the 4 groups will be separated by about 109.5° - this is the normal geometry for carbon.
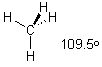
How to maximize repulsion? Separate by 120°.
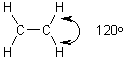 We have used 3 of the 4
electrons on carbon, the last electron is in a pz orbital -
these combine, on adjacent carbons, to make a PI bond (p
bond).
We have used 3 of the 4
electrons on carbon, the last electron is in a pz orbital -
these combine, on adjacent carbons, to make a PI bond (p
bond).
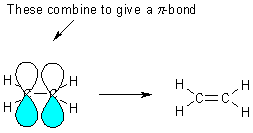
e.g., ethyne 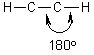
Note, there is still one electron in each of the py and pz orbitals (® total of 2 s bonds and 2 p bonds on each carbon)
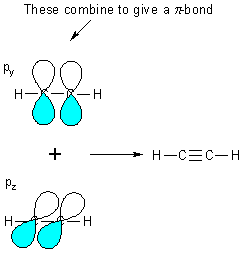
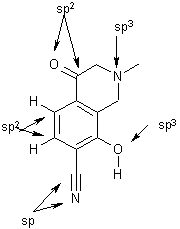
Other atoms participate in the same type of hybridization. See the examples below.
More Alkane Nomenclature
Rules for naming compound
1) Take longest linear chain (this gives "root")
2) number the molecule from one end to get lowest substitution numbers
3) name and number substituents
4) if more than one substitutent, use di, tri, tetra -
5) Arrange substituents in alphabetical order (excluding prefixes such as di, tri, i.e., triethyl precedes methyl)
Nomenclature - other functional groups
The groups that are arranged in alphabetical order are: halogens (Cl
chloro, Br bromo, I iodo) NH2 amino, CN cyano, NO2
nitro, and alkyl groups: methyl CH3 (Me), ethyl CH3CH2
(Et), propyl (Pr) CH3CH2CH2, isopropyl
(iPr) ![]()
Rotational isomers
Bond rotation along s -bonds takes place readily at room temperature. However, not all "twisted" structures are of equal energy. Generally, the most stable structures have big groups as far away from each other as possible. The repulsion of groups are called van der Waals interactions.
Let’s look first at a simple structure – ethane.
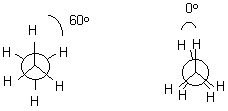
Staggered Eclipsed
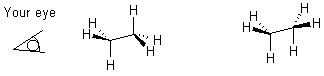
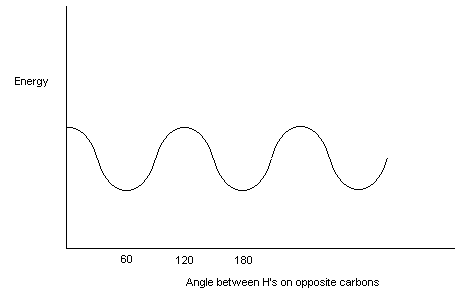
When there are more groups, the situation is a little more complex
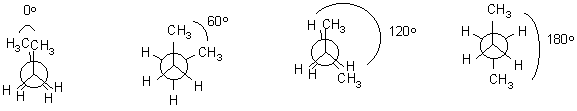
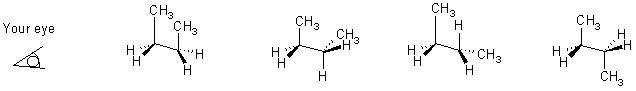
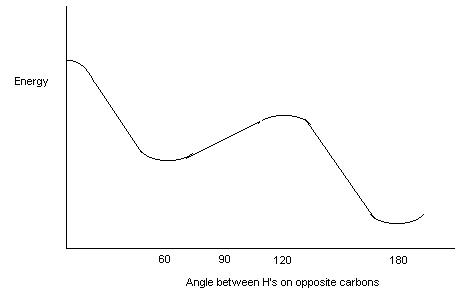
Other functional groups take a different priority (highest priority
at top, lowest at bottom and then the "alphabetical groups" after that.
| 1 | Carboxylic Acid |  |
Always C1 | ethan "oic acid" (acetic acid) |
| 2 | Ketone |  |
2-propan "one" (acetone) | |
| 3 | Aldehyde | 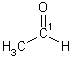 |
propan "al" (1-propanal) | |
| 4 | Alkene | H3CHC=CH2 | 1-prop "ene" (double bond starts at carbon 1) | |
| 5 | Alkyne | H3CCºCH | 1-propyne (triple bond starts at carbon 1) |
1) having the lowest number is most important - find longest chain, arrange substituents in alphabetical order and lowest substituent #
e.g. 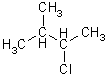
longest chain = 4 = butane
2) if same numbers, irrespective of which end you begin with, choose alphabetical and lower numbers,
i.e., 2-chloro-3-methyl-butane not 3-chloro-2-methyl butane
 4-bromo-2-chloro-4-methylhexane
is correct
4-bromo-2-chloro-4-methylhexane
is correct
3-bromo-5-chloro-3-methylhexane is not: the lowest number is higher in this name
Order of preference for naming
Highest
RCOOH > RCHO > 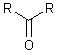 >
R3C-OH > RNH2 > R2C=CR'2
>
RCº CR'
>
R3C-OH > RNH2 > R2C=CR'2
>
RCº CR'
carboxylic acid aldehyde ketone alcohol amine alkene alkyne*
then come other substituents: halo, methyl, nitro, etc.
* note that this order is opposite in some books
Other Functional Groups
ETHER CH3CH2OCH3 ethyl methyl ether
AMINE CH3NHCH2CH3 ethylmethylamine
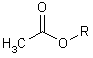 CH3CO2CH3
methyl ethanoate
CH3CO2CH3
methyl ethanoate
Cyclic Alkanes (CnHn+2)
Just add cyclo to name.

cyclopropane cyclobutane cyclopentane cyclohexane
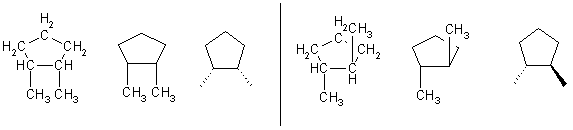
cis-1,2-dimethylcyclopentane trans-1,2-dimethylcyclopentane
Alkenes
ANE ® ENE
Naming ends is ene ethane ® ethene
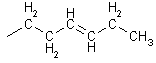 3 - heptene
3 - heptene
longest chain with double bond in it - gives double bond lowest possible number
i.e. 3-pentene not 4-pentene
If the groups with highest atomic number are on the same side - Z-isomer (zusammen), if on opposite sides – E-isomer (entgegen)
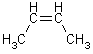
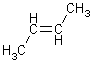
Z-isomer E-isomer
If 2 identical atoms go to next atom in chain; next structure is Z-1-bromo-2-pentene (put starting carbon of alkene at lowest number of chain)

Cyclic Alkenes

cyclobutene 4-methylcyclopentane
Triple bond – Alkyne
ANE ® YNE
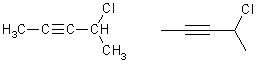
4-chloro-2-pentyne yne ending
Alcohol
ANE ® ANOL

4-bromo-2-pentanol
Ketone
ANE ® ONE ("OWN")
 ONE has precedence over
other groups listed above
ONE has precedence over
other groups listed above
3-chloro-2-butanone
Aldehyde
ANE ® ANAL
4-methylpentanal

Carboxylic Acids
ANE ® ANOIC ACID
ETHANOIC ACID (acetic acid) 3-BROMO-PROPANOIC ACID

Summary of Formulas & Isomers
1. Molecular Formula
C4H8 C3H8 These are clearly different
2. Structural isomers: Same molecular formula – different arrangement of groups, Stereoisomers have different properties
i.e., boiling point, melting point, etc.
e.g., C4H9Cl
2-chlorobutane 1-chlorobutane
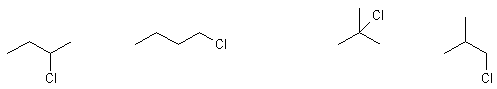
Cyclic alkanes Alkenes
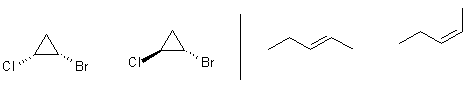
cis-1-bromo-2-chlorocyclopropane E-2-pentene
trans-1-bromo-2-chlorocyclopropane E-2-pentene
4. Rotational isomers
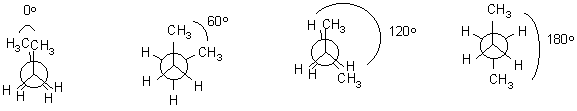

Alkanes: Properties and Reactions
| CnH2n+2 | b.p. |
m.p.
|
Increasing London |
| Forces (going down table) | |||
| CH4 |
-164
|
-182.5
|
|
| C2H6 |
-88
|
-183
|
|
| C3H8 |
-42
|
-190
|
|
| C5H12 |
36
|
-130
|
|
| C10H22 |
174
|
-30
|
|
| Polyethylene |
burns
|
140
|
|
| (C100H202) |
Tg~20
|
the chemistry of parent defines chemistry for the series
Homologous series each "homologue" 1 CH2 more
Natural gas = methane and some ethane, a little propane
Petroleum (black gold, texas tea)
Distillation gives
| Natural gas | C4 | < 20o |
| pet ether | C5 - C6 | 30-60 |
| Ligroin | C7 | 60-90 |
| Light naptha | C5-C9 | |
| Gasoline | C6 - C12 | 85-200 |
| Kerosene | C12-C15 | 200-300 |
| Loading oil | C15-C18 | 300-400 |
| Oil, general paraffin -> Asphalt | C16 - C20 | >400 |
Alkanes – other natural sources - "fart" produced in anaerobic bacterial decomposition (e.g., cow stomach (blue angels))
also found in salt mines, coal mines
Reactions of Alkanes
As we already saw, not very reactive
1) strong bonds C-C, C-H
2) not polar
C - H small polarization
Alkanes - cycloalkanes "paraffin" means unreactive
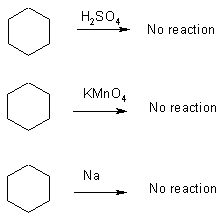
To decompose Na use alcohol in parafin – only the alcohol reacts
2Na + 2 CH3CH2OH ® 2 CH3CH2ONa+ + H2
1 Halogenation with Cl2, or Br2
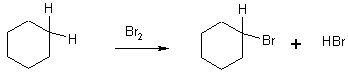
This is a radical chain reaction – doesn’t work in the dark
3 parts Initiation, Propagation, Termination
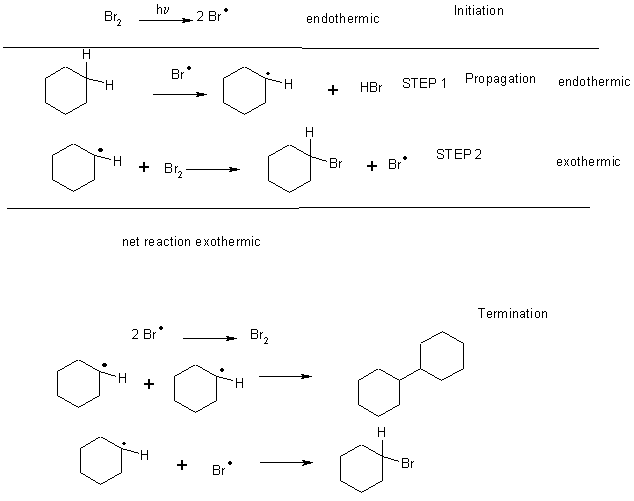
Let’s look at a simpler system
CH4 + Cl2 ® CH3Cl + HCl D Hrxn = -104 kJ mol-1
1) Cl2 ® 2 Cl·D H1 = +243 kJ mol-1
2) Cl· + CH4 ® CH3· + HCl D H2 = +4 kJ mol-1
3) CH3· + Cl2® CH3Cl + Cl·D H3 = -108 kJ mol-1
But for bromination
CH4 + Br2 ® CH3Br + HBr D Hrxn = -34 kJ mol-1
1) Br2 ® 2 Br· D H1 = +192 kJ mol-1
2) Br· + CH4 ® CH3· + HBr D H2 = +66 kJ mol-1
3) CH3· + Br2® CH3Br + Br· D H3 = -100 kJ mol-1
Note that chlorination is mildly endothermic in the first step of propagation (step 2) whereas bromination is quite endothermic. In the second step of the propagation, both are exothermic. The overall reaction rate is dependent upon the activation energy in the slowest step (step 2). The Ea for chlorination is much lower that for bromination, which one might predict from the overall enthalpies of the steps.
The overall reaction with Cl2 is faster than Br2
If excess halogen, e.g., Cl2, more chlorination
i.e. CH4 ® CH3Cl ® CH2Cl2
But for I2 step 1 very endothermic + 200 kJ/mol
\ reaction very slow so not useful
For F2, steps 1 and 2 are very exothermic step a ~ -144 kJ/mol ® explosive reaction
\ use other reagents to make F- alkanes (CoF3, SF4)
If one uses unsymmetical alkanes, there are different types of CH bonds. Depending on which bond reacts, different products are formed. The preference depends on the strength of the C-H bonds and on the number of hydrogens of a given type. In general, effects from both factors are observed.
The bond strengths of CH bonds depend on the number of carbons connected
to the central carbon.
| Reactant | Products | Bond Dissociation kJ mol-1 |
| H3CH | H3C· | 426 |
| H3CCH2H (Bold carbon is primary) | H3CC·H2 a primary radical | 405 |
| (H3C)2CHH (Bold carbon is secondary) | (H3C)2C·H a secondary radical | 397 |
| (H3C)3CH (Bold carbon is tertiary) | (H3C)3C·a tertiary radical | 376 |
The ease of forming a carbon radical (and the order of highest stability) is
3° (tertiary) > 2° (secondary) > 1° (primary) > methyl
Recall for chlorination (and bromination)
RH + Cl· ® R· + HCl endo (slow)
R + Cl2 ® RCl + Cl· exo
What happens in a molecule with both types of hydrogens – both happen
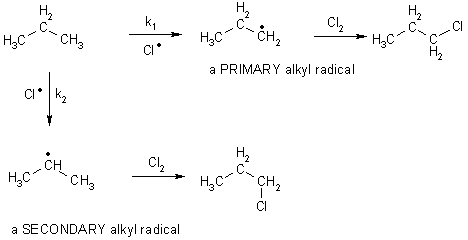
The rates are proportional not only to the bond strength of the CH bond being broken, but also on the statistical number of hydrogens. The bond strength is the most important factor. (i.e., generally k2 > k1)
Rate of reaction via 1° CH = k1 [CH3CH2CH3][Cl· ] x fn 6 H’s
Rate of reaction via 2° CH = k2 [CH3CH2CH3][Cl· ] x fn 2 H’s
Where fn is some fractional effect of arising from the statistics.
The product ratio between these two products will be rather similar to k1 / k2
The actual ratio of products is 1° alkyl halide 45%, 2° alkyl halide 55%
2 Alkanes - Combustion
This is the fundamental reaction of the 20th century
CnH2n+2 ® (3n +1)/2 O2® nCO2 + (n+1) H2O + heat
H4C + 2 O2 ® CO2 + 2 H2O + D
e.g., cigarette lighter C4H10 + 6.5 O2®
4CO2 + 5H2O
| Alkane | DH combustion (kJ/mol) |
| CH4 | 213 |
| H3CCH3 | 373 |
| C4H10 | 687 |
| 656 Ring strain | |
| C5H12 | 845 |
 (C5H10) (C5H10) |
793 |
Alkyl Halides ? Haloalkanes
Chemistry controlled by bond polarity
d d -C-Hdd+d+C-Xd- X = F high dipole moment ® X = I lowest polarity
\ alkyl halides have higher m.p. & b.p.’s than related alkanes that only have London forces
CH4 b.p. -164 ° C H2CCl2 b.p. 35 ° C
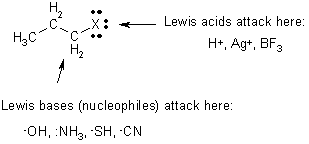
Reactions
1 Haloalkane Preparations; From alkanes
Seen above
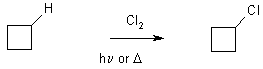
3 From alcohols
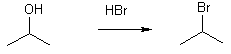
2-propanol a substitution reaction
4 Addition to alkene

Reactions
5 Nucleophilic Substitution
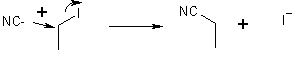
Many e.g.’s of substitution reactions. (For OH- need dilute solutions, see below)
6 Elimination of HX
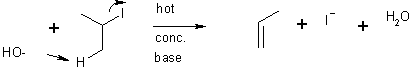
7 Reactions with Group 1 or 2 metals Li / Mg

= Organometallic
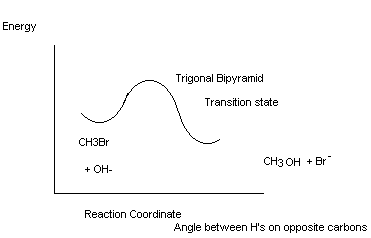
SN2 Reactions
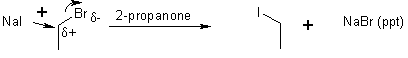
Kinetics process of process - d[EtBr]/dt = k[EtBr]1[I-]1
Second order – bimolecular; these kinetics are observed for MeX, 1° RX (RCH2X) and 2° RX (RR'CHX) BUT NOT FOR 3° RX (RR'R"CX)
Called SN2 substitution nucleophilic bimolecular
Go from 1 isomer to a different isomer; i.e., Inverted stereochemistry at the reaction centre
Most nucleophilic substitutions take place this way.
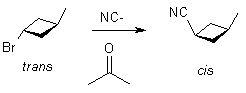
Mechanism
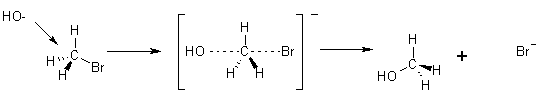
The SN1 Reaction
For 3° alkyl halides need very polar solvents and non basic nucleophiles to observe nucleophilic substitution. However, the kinetics are different.
(CH3)3CBr + N3_ ® (CH3)3C-N3
a first order reaction; implies the overall reaction is not an elementary step (that the rate detemining step doesn’t need a nucleophile).
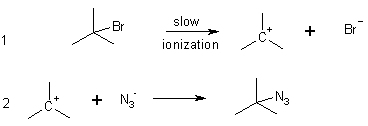
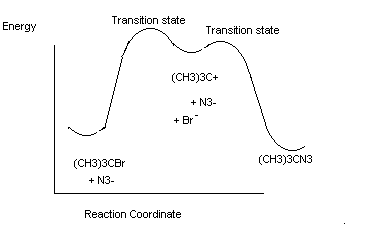
Why doesn’t the SN2 happen with 3° alkyl halides? Two reasons – i) Steric reasons (space occupied). The alkyl groups block backside attack. ii) the 3° cation is sufficiently stable that another reaction pathway exists.
Order of stability of carbocations:
3° ((CH3)3C+) > 2° ((CH3)2C+H) > 1° (H3CC+H2) > H3C+
Why? The alkyl groups help stabilize the cation by donating electronic charge. Smearing out charge is energetically favourable.
One can accelerate the rate of these reactions using Lewis acids. Silver removes the chloride in tertiary chlorides to help form the cation.
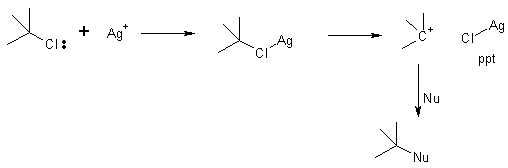
This is a useful chemical test for 1° 2° and 3° alkyl halides
Some nucleophilic substitutions
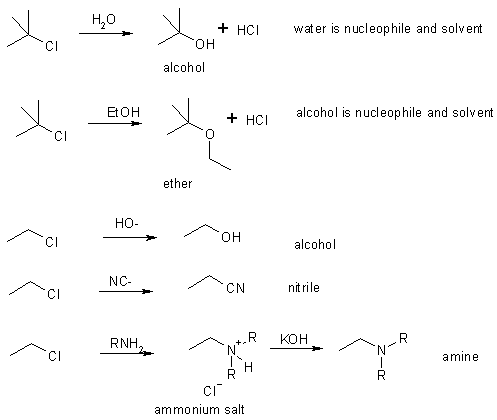
Summary of Substitution
| Methyl | 1° | 2° | 3° | |
| CH3X | C-CH2X | C2CHX | C3CX | |
| SN2 relative rate | 30 | 1 | 0.03 | 1 x 10-6 |
| SN1 | H3C+ never see | C-CH2+ almost never see | 1-10% of SN2 rate | 100% |
Elimination
We saw above that OH- + an alkyl halide gives an alcohol. This is true only if COLD DILUTE OH- (KOH, NaOH) is used. If HOT CONCENTRATED (e.g., > 3M) is used, a second order ELIMINATION occurs to give an alkene.
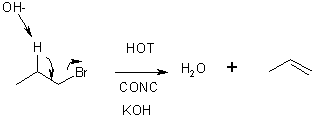
In this reaction, the HO- is acting as base, not a nucleophile.
Rate Law; Rate = k[RX][OH-]; called E2 (elimination bimolecular)
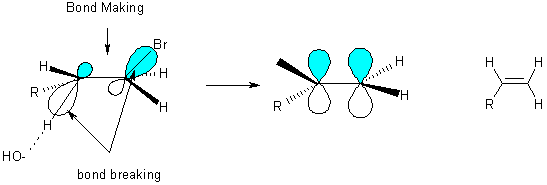
Generally the more carbon groups on a double bond, the more stable it is: Saytzeff’s rule
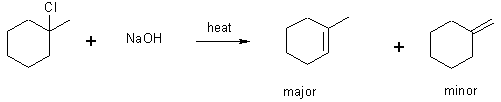
7 Organometallics
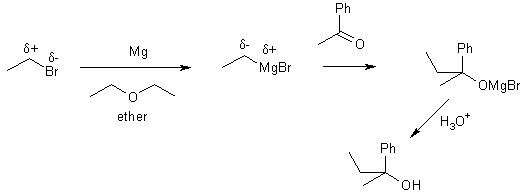
We can completely alter the electronic distribution in a molecule by converting an alkyl halide into an organometallic compound.
H3Cd+-Id- + Mg ® H3Cd--Mgd+-I methylmagnesium iodide

These are carbanions (very strong bases and nucleophiles). This is one of the few reactions we learn from which C-C bonds can be formed.
8 Reaction with water
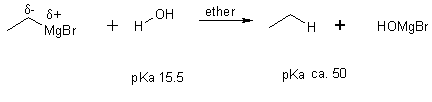
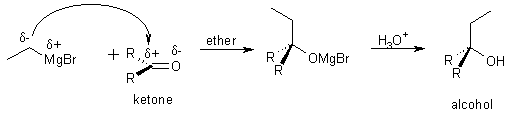
9 Reaction with ketones or aldehydes
10 Reaction with CO2

Alkenes
Preparation
6 From Haloalkanes
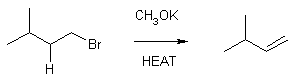
1-bromo-3-methylbutane 3-methyl-1-butene
11 From alcohols
an alcohol + an dehydrating acid (H2SO4, H3PO4)
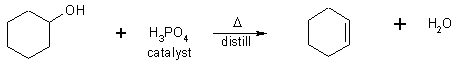
cyclohexanol catalysts cyclohexane
b.p. 156 ° C b.p. 82 ° C
Alkene Reactivity
Dominated by p bonds
p bond \ energy (~ 267 kJ/mol strength of the double bond) more reactive than a s -bond ( 310 kJ/mol); It is a Lewis base - attack by E+ on p-electrons, i.e. In plane of screen above or below p -bond
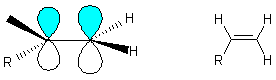
These are extremely reactive almost as reactive as the metal
Fats: in the body: triglycerides
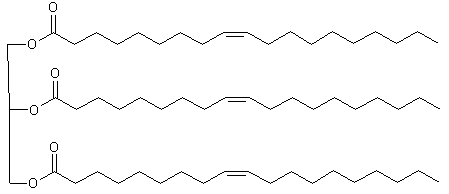
| 1 | 2 | 3 | ||||||
| Oleic | linoleic | limolenic | ||||||
| C12 | C14 | C16 | C18 | C18 | C18 | C18 | ||
| beef fat | 0 | 0 | 27 | 14 | 49 | 2 | - | |
| lard | 0 | 1 | 24 | 9 | 47 | 10 | 0 | |
| human | 1 | 3 | 27 | 8 | 48 | 10 | - | |
| leving | 0 | 5 | 14 | 3 | 0 | 0 | 30 | |
| corn | 0 | 1 | 10 | 3 | 50 | 34 | 0 | |
| olive | 0 | 0.1 | 7 | 2 | 84 | 5 | 0 |
Other important alkenes: squalene ® cholesterol; b -carotene ® retinal (vision)
b -selinene, celery oil
myrcene, bay leaf oil
a -pinene, cedar leaf oil
Alkenes may exist in two different geometric isomers (see above) (Z- and E-)

Z-2-butene E-2-butene
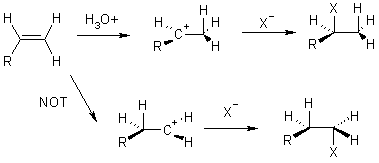
Alkene Reactions
Electrophilic additions
Remember that the order of cation stability is:
3° ((CH3)3C+) > 2° ((CH3)2C+H) > 1° (H3CC+H2) > H3C+
4 Acids and 12 alcohols
additions to alkenes usually proceed via the most stable cation.
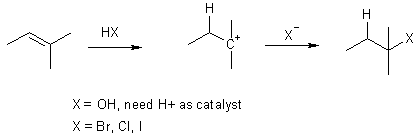
get both cis and trans addition
13 Bromination
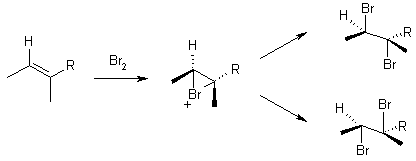
Can also use ICl (I+ Cl-)
Only get trans addition

14 Reduction
In organic chemistry, addition of H’s or removal of oxygen is "reduction". The removal of hydrogens or addition of oxygen is "oxidation". Conversion of C=C to HC-CH is a reduction
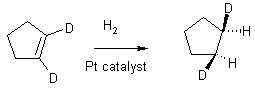
cis-addition of H2, the reaction happens at the solid surface of Pt
Process used commercially to "hydrogenate" fats.
Oleic acid
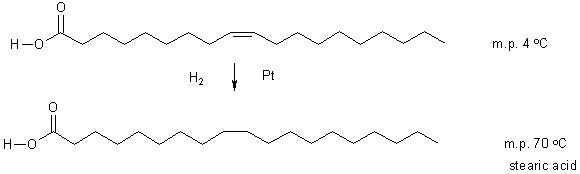
Stearic acid is a saturated fat;
Not so good for you. Better for your health are polyunsaturated fats.
Alcohols & Ethers
Unlike the functional groups we have seen so far, alcohols and ethers (to a lesser extent) are polar molecules. In the case of alcohols, there is strong H bonding and reasonably large dipole moments.

Alcohols Preparation
5 SN2 of alkyl halides with hydroxide
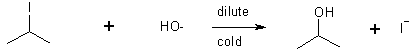
12 Hydration of alkenes
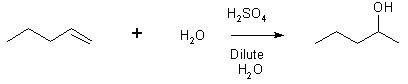
9 addition organometallic to aldehyde or ketone
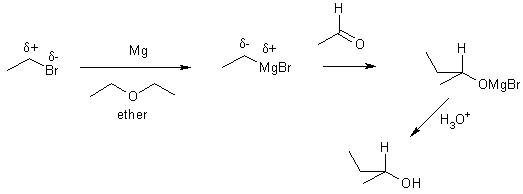
electrophilic Nucleophilic
CH3I + 2 Li ® CH3Li + LiI
From electronegativity, the polarization (and reactivity) of a ZM bond, M = Na, Li, Z = first row elements follows the reactivity
-CH3 > -NH2 > -OH > -Cl
Any reaction that converts one of the compounds on the left to one on the right will be thermodynamically favoured.
e.g., CH3Li + H-OH ® CH3-H + LiOH
Less stable More stable
CH3MgBr + H-OH ® CH3-H + BrMgOH
Other C-C Bond Forming Reactions

Note: CAN’T DO THE FOLLOWING REACTION.
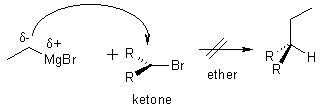
Ethers are less polar than the alcohols (No OH’s for H-bonding)
They are made in a similar fashion to alcohols
5 Preparation of ethers
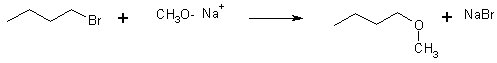
15 Making Alkoxides (Reducing Metals)
![]()
Alkoxides are strong bases and also good Lewis bases or nucleophiles
Recall: 2HO-H + 2K ® 2HO-K + H2
Reactions of Alcohols
3 Preparation of alkyl halides
Alcohols & halogen acids e.g. HCl, HBr, HI

Note that the reaction doesn’t work under basic conditions
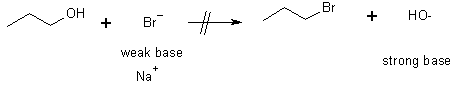
The mechanism
Step 1 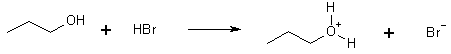
Step 2 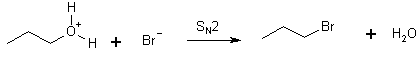
11 Alcohol & Dehydrating Acid
e.g. 80% H2SO4, or H3PO4 is required

cyclopentanol Elimination
16 Alcohols + Oxidizing Agents
One can use inorganic salts to oxidize (remove H’s or introduce oxygen) onto organic molecules.
eg. CrO3 or K2Cr2O7/H2SO4
Chromium IV or Na3Cr2O7/H2SO4 º H2CrO4 or KMnO4 (MnVII)
Chromic acid
Or "Organic Chromium Salts, like pyridine chlorochromate (PCC) 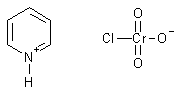
1o Alcohol C - CH2OH
(a) with PCC
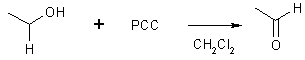
removes this H Ethanol (acetaldehyde)
(b) with K2Cr2O7/H+ or KMnO4
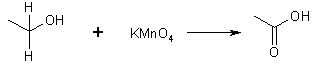
2o Alcohol + Any oxidant

2-propanol 2-propanone
3o Alcohol
no reaction at normal temps, No H-COH to oxidize!
Aldehydes & Ketones
Preparation
16 Oxidation of 1o Alcohol
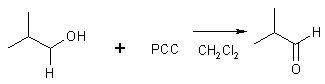
2-methylpropanol 2-methylpropanal
(2) Oxidation of 2o Alcohol
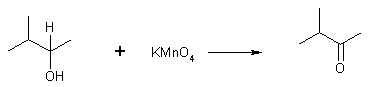
3-methyl-2-butanol 3-methyl-2-butanone
Reactions of Aldehydes and Ketones
Nucleophilic Addition
The carbon in C=O is electron-poor, an electrophile.
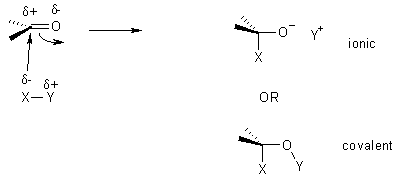
Examples
9 Organometallic + Ketone
aldehyde ® secondary alcohol
ketone ® alcohol
16 Reduction with NaBH4
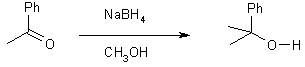
17 Reduction with H2 and a P+ catalyst
NOTE: This is exactly the same as addition of H2 to an alkene
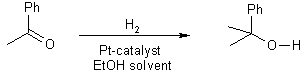
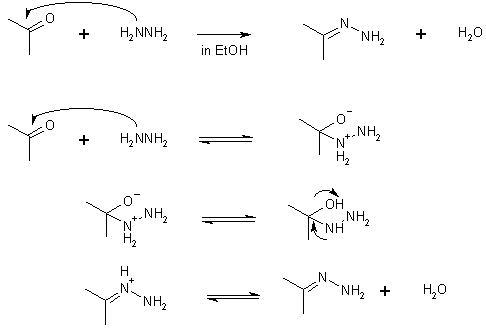
18 Reaction with N-Compounds
e.g., hydrazine H2NNH2; 2,4-dinitrophenylhydrazine  ;
hydroxylamine H2NOH
;
hydroxylamine H2NOH
16 Oxidation - Aldehydes
Occurs very easily, while can use strong oxidants such as K2Cr2O7/H+ or KMnO4
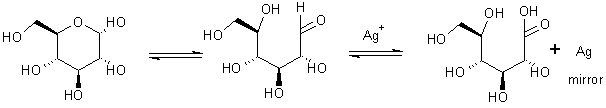
Can also use weak oxidizing agents eg. Ag
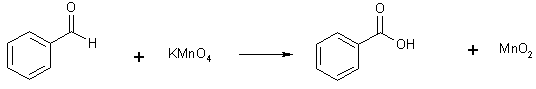
Another more relevant oxidant – how to make a mirror
NOTE: Ketones + oxidants No reaction at normal temps
Carboxylic Acids
Structures: Pure glacial acetic acid Methanoic acid (formic acid)

Ethanoic acid
The Structure Type
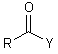 Is very common
Is very common
R = any alkyl or aryl (aromatic)
e.g. 
Amides Esters Acid chlorides acetic anhydride
Preparation of Carboxylic Acids
10 Organometallic + CO2
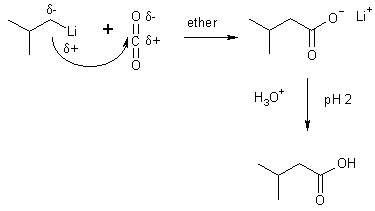
(2-Methylpropyl)lithuim
16 Aldehyde + any oxidant
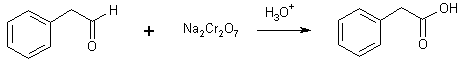
2-phenylethanal 2-phenylethanoic acid
(Phenyl acetic acid)
1o Alcohol + Any oxidant
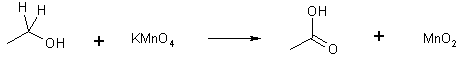
ethanol ethanoic acid (acetic acid)
20 Reaction of -COOH with an Alcohol ® Esters
Need a strong acid catalyst
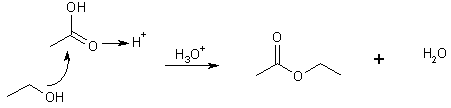
ethanoic acid ethyl ethanoate (ethyl acetate)
Reaction is a Nuc. Add followed by an elimination
(2) Acidity pKa» 3- 5
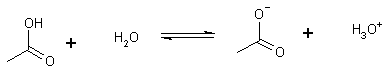
ethanoic acid
(acetic acid)
-two O atoms and therefore the O-H bond is weakened
moreover, there is resonance through which the charge is dispersed.
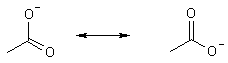
Compare Alcohols
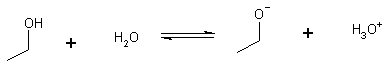
Only one O to share electrons pKa » 16-18
NOTE: an alkane C-H is an unbelievably weak acid, nothing to stabilize the charge
CH4 ® CH3- + H3O+
ie. pKa of ca. 50