
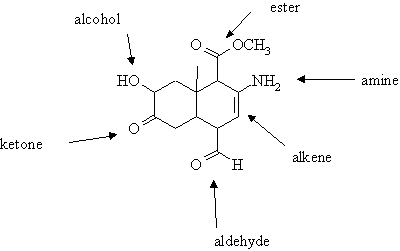
2. Name all the functional groups in the following molecule.
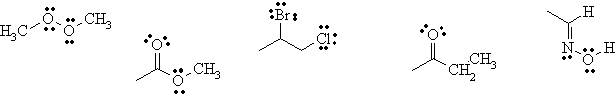
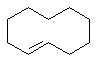 ii)
ii) 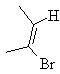 iii)
iii) 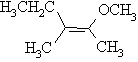
E-cyclodecane E-2-bromobut-2-ene
Z-2-methoxy-3-methylpent-2-ene
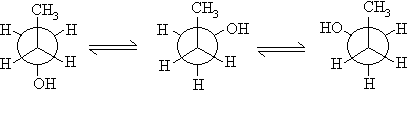
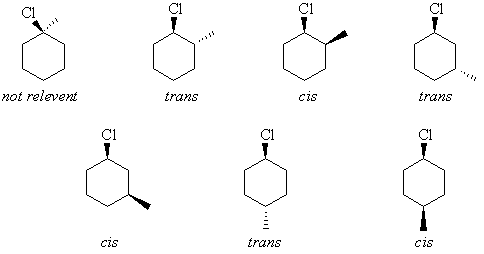
The anti is more stable.
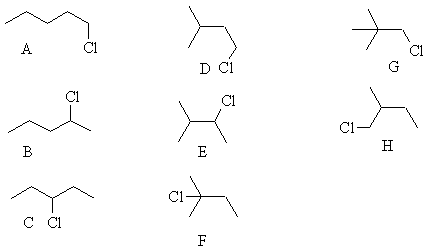
i) Draw their structures
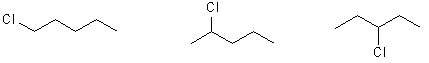
ii) give their names
1-chloropentane 2-chloropentane 3-chloropentane
iii) predict the ratios of the three products remembering that a chlorine
abstracts a secondary H![]() (CH2)
about 4 times faster than a primary H
(CH2)
about 4 times faster than a primary H ![]() (CH3).
(CH3).
You need to consider the statistical number of hydrogens and the relative
rate with which they will react. We know k1 =k2 /4
The free radical chlorination of alkanes involves 3 steps.
Initiation: Cl2 ® 2 Cl![]()
Prop HCR3 + Cl![]() ®
HCl + HC
®
HCl + HC![]() R2
R2
HC![]() R2 + Cl
2® HCR2Cl + Cl
R2 + Cl
2® HCR2Cl + Cl![]()
Term Cl![]() + Cl
+ Cl![]() ®Cl
2
®Cl
2
Cl![]() + HC
+ HC![]() R2®HCR2
Cl
R2®HCR2
Cl
HC![]() R2 + HC
R2 + HC![]() R2
+
®
HCR2CR2H
R2
+
®
HCR2CR2H
The important question is whether CH2 of CH3 is
more reactive. We learned that a 2o radical is more stable than
a 1o radical. Also important is the statistical differences
between 2 x CH3 group, 2 x CH2 groups (C2), and 1
x CH2 groups (C3)
But k1 = k2 / 4 and there is the statistical difference in the numbers of hydrogens present to react.
(6H for the Me groups versus 4H for the (C2) CH2 groups
and 2H for the (C3) CH2).
Rate producing 1-chloropentane = k1 [pentane] * number of primary hydrogens
Rate producing 2-chloropenane = k2 [pentane] * number of secondary hydrogens on C2
Rate producing 3-chloropenane = k2 [pentane] * number of
secondary hydrogens on C3
Fraction that reacts to give 1-chloropentane = k1 *6
Fraction that reacts to give 2-chloropentane = k1 * 4 *4
Fraction that reacts to give 3-chloropentane = k1 * 4 *2
sum of fractions = 1 (all pentane reacts to give a monochloropentane)
1 = k1 *6 + k1 * 4 *4 + k1 * 4 *2 k1=
1/(6+16+8) = = 1/30 = 0.033
Therefore amount of 1-chloropentane = k1 *6 = 0.033 * 6 = 20%
Therefore amount of 2-chloropentane = k1 * 4.5 *4 = 0.033 * 16 = 53%
Therefore amount of 2-chloropentane = k1 * 4 *2 = 0.033 *
8 = 26%