(a) Solid A was found to decompose to gaseous products on warming. At 220K A had a half-life of 6 hours while at 250K only 10% of the original sample remained after one hour. Calculate the activation energy for the decomposition of A.
(b) On addition to water a sample of solid A produced HF and 4.19 g of Kr(g). On dilution to 1.00 L, a solution with a pH of 2.23 was obtained. What is the empirical formula of solid A?
2. Draw clear structural diagrams for:
(a) all the isomers which satisfy the formula C4H8Cl2,
(b) all the cycloalkane isomers which satisfy the formula C4H6Cl2.
(Do not name any of these compounds.)
3. Draw clear structural diagrams of each of the following molecules to illustrate their three-dimensional natures (see Figure 2.2 in Organic Notes):
(a) butane (d) cyclopentane
(b) 1,3-dibromopropane (e) trans-1,3-dichlorocyclobutane
(c) 2-methylbutane (f) cis-1,2-diethylcyclopropane
4. Benzo[a]pyrene is a hydrocarbon and a carcinogen (a cancer-causing substance) which is produced during the combustion of wood, coal, diesel fuel, gasoline and tobacco.
(a) Calculate the empirical formula of benzo[a]pyrene given that this molecule is 95.2% carbon by weight.
(b) The molecular mass of benzo[a]pyrene was determined to be 252 g/mole. What is the molecular formula?
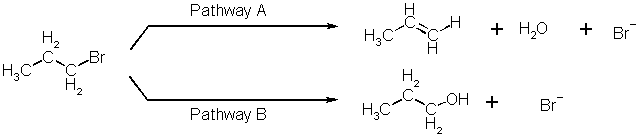
5. 1-Bromopropene can react with hydroxide ion via two separate reaction pathways (A and B) to give two different organic reaction products, propene and 1-propanol.
Reactions proceeding via pathway A to propene have an activation energy of 245 kJ/mole while reaction proceeding via pathway B to 1-propanol have an activation energy of 180 kJ/mole. At 65 oC the two pathways have identical rates. The two pathways also have identical rate laws given by:
Rate = k [1-bromopropane] [OH-]

(a) What is the ratio of products (propene:1-propanol) when equimolar amounts of 1-bromopropane and hydroxide are reacted at 65 oC?
(b) The 65 oC reaction was repeated at 100 oC. What is the ratio of products (propene:1-propanol) at 100 oC?
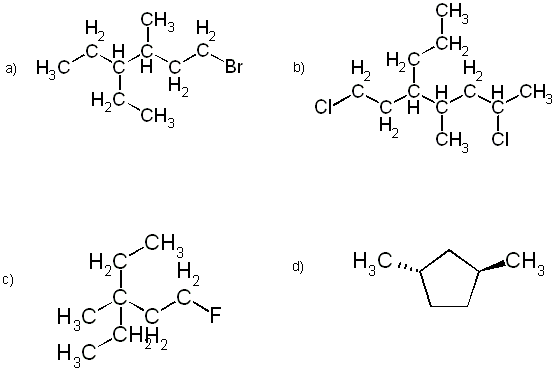
6. Name the following molecules according to the IUPAC system of nomenclature: