1
HCl + HCl![]() H2Cl2
H2Cl2
(fast equilibrium)
2
HCl + H3C-CH=CH2 ![]() H3C-CHClCH3
H3C-CHClCH3
(fast equilbrium)
3
H3C-CHClCH3
* + H2Cl2(slow)
___________________________________________
1. The decomposition of hydroxylamine (H2NOH) in the presence of oxygen follows the rate expression:
Rate = kobs [H2NOH][O2]
where kobs = 0.237 x 10-4 L/mol s at 0 oC and 2.64 x 10-4 L/mol s at 25 oC. Calculate the activation energy for this process and the Arrhenius factor A for this reaction.
2. HCl reacts with propene (H3C-CH=CH2) in the gas phase according to the overall reaction.
HCl + H3C-CH=CH2
The experimental rate expression is
rate = k [HCl]3 [H3C-CH=CH2]
A suggested reaction mechanism is
|
1 |
HCl + HCl |
(fast equilibrium) |
|
2 |
HCl + H3C-CH=CH2 |
(fast equilbrium) |
|
3 |
H3C-CHClCH3 * + H2Cl2 |
(slow) |
* refers to an activated molecule.
Show that these steps are consistent with the experimentally observed rate law.
3. The rate constant of the elementary reaction
BH4- (aq) + NH4+ (aq) ![]() BH3NH3 (aq) + H2 (g)
BH3NH3 (aq) + H2 (g)
is k = 1.94 x 10-4 L/mol s at 30.0 oC and the reaction has an activation energy of 161 kJ/mol.
a) Calculate the rate constant of the reaction at a temperature of 40 oC.
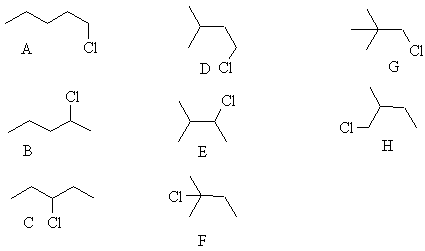
4. Draw line structures and name 5 of the structural isomers of molecular formula C5H11Cl.
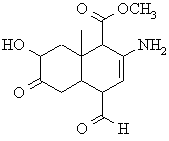
5. Name all the functional groups in the following molecule.
6. Write Lewis structures (showing all lone pairs) for the following:
i) CH3O2CH3 ii) HCO2CH3 iii) BrCHCH3CH2Cl iv) CH3COC2H5 v) CH3CHNOH