| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 11. Proteins and Carbohydrates. Isolation of Casein and Lactose from Milk.
References: Ege, Chapter 25,26
Background
Milk is the most nutritionally complete food found in nature. All kinds of milk, human or animal, contain vitamins (principally thiamine, riboflavin, pantothenic acid, and vitamins A, B12, and D), minerals (calcium, potassium, sodium, phosphorus, and trace metals), proteins (mostly casein), carbohydrates (principally lactose), and lipids (fats). The amounts of these nutrients present in different types of milk differ greatly, however. Cows' milk and goats' milk are almost identical in every respect. Human milk contains less than half of the proteins and minerals of cows' or goats' milk, but almost 1.5 times as much sugar. Horses' milk is quite low in proteins and fats compared with the others, whereas reindeer milk is very high in proteins, fats, and minerals, but quite low in carbohydrates. The average composition of whole cows' milk is 87.1% water, 3.4% protein, 3.9% fats, 4.9% carbohydrates, and 0.7% minerals. The only important nutrients lacking in milk are iron and vitamin C.
Whole milk is an oil-in-water emulsion, containing its 3.9% fat dispersed as micron-sized globules. The fat emulsion is stabilized by complex phospholipids and proteins that are adsorbed on the surfaces of the globules. Because the fat in milk is so finely dispersed, it is digested more easily than fat from any other source. The globules are lighter than water, and thus coalesce on standing and eventually rise to the surface of the milk as cream. Vitamins A and D are fat-soluble substances and are thus concentrated in the cream. The fats in milk are primarily triglycerides, which are esters of saturated and unsaturated carboxylic acids with glycerol, a tri-alcohol. About two thirds of the fatty acids in milk are saturated, and consist primarily of C12, C14, and C16 acids. Milk is unusual in that about 12% of the fatty acids are short-chain fatty acids (C2-C10) like butyric, caproic, and caprylic acids. Additional lipids (fats and oils) in milk include small amounts of cholesterol, phospholipids, and lecithins. The phospholipids help to stabilize the whole milk emulsion, as the phosphate groups help to achieve partial water solubility for the fat globules. All the fat can be removed from milk by extraction with petroleum ether or a similar organic solvent.
There are three kinds of proteins in milk: caseins, lactalbumins, and lactoglobulins. All three are globular proteins, which tend to fold back on themselves into compact, nearly spheroidal units and are more easily solubilized in water as colloidal suspensions than fibrous proteins are. They are "complete proteins", so-called because they contain all the amino acids essential for building blood and tissue, and they can sustain life and provide normal growth even if they are the only proteins in the diet. These proteins not only contain more amino acids than plant proteins, but they contain greater amounts of amino acids than the proteins in eggs and meats.
Casein, the main protein in milk, is a phosphoprotein, meaning that phosphate groups are attached to the hydroxyl groups of some of the amino acid side-chains. Casein exists in milk as the calcium salt, calcium caseinate. It is actually a mixture of at least three similar proteins which differ primarily in molecular weight and the amount of phosphorus groups they contain. Alpha- and beta-casein have molecular weights in the 25,000 range and possess about 9 and 4-5 phosphate groups per molecule, respectively. They are both insoluble in water. Kappa-casein has a molecular weight of about 8,000 and possesses 1-2 phosphate groups per molecule. It is responsible for solubilizing the other two caseins in water by promoting the formation of micelles.
Calcium caseinate has an isoelectric point of pH 4.6. Therefore, it is insoluble in
solutions of pH less than 4.6. The pH of milk is about 6.6; therefore, casein has a
negative charge at this pH and is solubilized as a salt. If acid is added to milk, the
negative charges on the outer surface of the casein micelles are neutralized (by
protonation of the phosphate groups) and the neutral protein precipitates, with the
calcium ions remaining in solution:
Ca-caseinate + 2H+ ---> casein + Ca2+
A natural example of this process occurs when milk sours. The souring of milk is an intricate process started by the action of microorganisms on the principal carbohydrate in milk, lactose. The microorganisms hydrolyse the lactose into glucose and galactose. Once galactose has been formed, lactobacilli, a strain of bacteria present in milk, convert it to the sour-tasting lactic acid. Since the production of the lactic acid also lowers the pH of the milk, the milk clots when it sours due to the precipitation of casein.
When the fats and proteins have been removed from milk, the carbohydrates remain in the
whey, as they are soluble in aqueous solution. The main carbohydrate in milk is lactose.
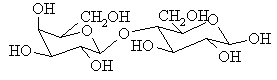
Lactose (4-O-(![]() -D-galactopyranosyl)-D-glucopyranose) is the only
carbohydrate that mammals synthesize. It is a dissacharide consisting of one molecule of
D-glucose and one molecule of D-galactose joined in 1,4'-fashion, and is synthesized in
the mammary glands. In this process, one molecule of glucose is converted to galactose and
joined to another of glucose. Galactose is thought to be needed by developing infants to
build brain and nervous tissue. It is more stable to metabolic oxidation than glucose and
affords a better material for forming structural units in cells. The digestion of lactose
involves the enzyme lactase, which hydrolyzes the disaccharide into its two component
sugars.
-D-galactopyranosyl)-D-glucopyranose) is the only
carbohydrate that mammals synthesize. It is a dissacharide consisting of one molecule of
D-glucose and one molecule of D-galactose joined in 1,4'-fashion, and is synthesized in
the mammary glands. In this process, one molecule of glucose is converted to galactose and
joined to another of glucose. Galactose is thought to be needed by developing infants to
build brain and nervous tissue. It is more stable to metabolic oxidation than glucose and
affords a better material for forming structural units in cells. The digestion of lactose
involves the enzyme lactase, which hydrolyzes the disaccharide into its two component
sugars.
In the first part of this experiment, we will isolate casein and lactose from cows' milk and carry out a few chemical tests on the isolated casein and lactose. As implied above, these are rather simple operations to carry out. Casein is precipitated by simply adjusting the pH of the milk to be sufficiently acidic that the protein is insoluble, taking care not to acidify too much so that the lactose does not hydrolyze. The other proteins remain water-soluble in acidic solution, but they can also be precipitated and isolated by merely heating the acidic solution and filtering. The isolated casein is insoluble in water, alcohol, and ether, but dissolves in alkaline and some acidic solutions. Once the casein is removed, lactose can be isolated as the alpha-anomer by addition of ethanol and crystallization from the resulting water-ethanol mixture at room temperature.
Casein is isolated from milk commercially and is industrially important because after dissolving in alkaline solutions and drying, it becomes a sticky substance that can be used in glues, the coating of paper, and the binding of colours in paints and wallpaper. It is also used as a coating for fine leather, and is cured with rennet to produce a plastic material used for buttons. When isolated under sanitary conditions and dissolved in alkaline solutions, casein is also employed in the manufacture of pharmaceutical and nutritional products.
In the test section of the experiment, we will carry out a few chemical tests on the isolated casein and lactose, as well as on test samples of other representative amino-acids and carbohydrates. Historically, these tests were designed for the purpose of structure elucidation. Since we already know the structures of these substances, we will use the chemical tests to demonstrate various aspects of the chemical reactivity of the protein casein. Of course, these tests depend on the specific structural features present in the molecules. While the tests and the chemistry involved are described briefly below, you should read Ege, Chapters 25 and 26 for a more complete introduction and the necessary background.
A. Isolation of Casein and Lactose from Milk
Isolation of Casein
Procedure: Weigh out 5 grams of powdered non-fat dry milk and dissolve it in 20 mL of warm water in a 100 mL beaker. Bring the temperature of the solution to 55oC (do not exceed) on a hot plate, remove the thermometer, and then add dropwise a solution of 10% acetic acid while stirring with a stirring rod. Do not add the acid too rapidly. Continue the acid addition (slightly less than 2 mL will be required), keeping the beaker on the hot plate, until the liquid changes from milky to almost clear and the casein no longer separates. It is important not to add too much acid, because it may hydrolyze some of the lactose in the milk and reduce your yield in Experiment 11B. Stir the precipitated casein until it forms a large amophous mass; then remove it with a stirring rod or tongs and place it in another beaker.
Immediately add 0.75 grams calcium carbonate to the original beaker containing the remaining liquid, stir for a few minutes, and save the resulting mixture for the later separation of lactose below. The separation of lactose should be done as soon as possible during the same laboratory period.
Collect the casein by suction filtration to remove as much water as possible. Press the solid with a spatula. Place the casein in a 100 mL beaker and add 5 mL of a mixture of 1:1 ethyl ether and ethanol (CAUTION: HIGHLY FLAMMABLE - NO FLAMES). Stir the casein in the ether for a few minutes, decant the ether, and repeat the process with a second 5 mL portion of ether. After the second washing with ether, suction filter the product. The ether washings remove any small quantities of fat that may have precipitated with the casein. Place the casein between several layers of paper towels to help dry the product, and let it stand in the air for 10-15 minutes. Divide the wet product in half, and weigh the two portions. Place one portion in a 125 mL Erlenmeyer flask with 35 mL of water and 0.5 mL of 1M NaOH, stopper the mixture, shake it to ensure solution of as much of the casein as possible, and save it for use in the chemical tests below. (You may carry out the chemical tests for the protein during this lab period if you have time, or in your next lab period.) Allow the second portion to dry in your locker over the following two weeks. When dry, weigh this portion and calculate the total yield of casein from the powdered milk. Show your calculations.
Isolation of Lactose
Procedure: Gently boil the original liquid to which the calcium carbonate was added after isolation of casein. Bumping will not be a problem so long as you stir the solution constantly and vigorously with a glass rod. The solution will foam somewhat as it refluxes. This procedure precipitates the remaining proteins lactalbumin and lactoglobulin. Suction filter the hot mixture to remove the proteins and calcium carbonate, and transfer the hot, slightly yellow filtrate to a 125 mL Erlenmeyer flask. Concentrate the filtrate to a volume of about 5 mL by heating with constant swirling, again being careful to avoid bumping. Foaming can be controlled by heating the liquid less vigorously and gently blowing onto it.
To the hot, concentrated solution, add 25 mL of hot 95% ethanol and 0.2 gram of decolourizing carbon. Put this mixture aside and prepare a slurry of about 1 gram of Celite and 7.5 mL of 95% ethanol. Suction filter the slurry into a Hirsch funnel containing a correct sized filter paper to obtain a filter pad of Celite, and discard the alcohol in the filter flask. [The Celite filter pad helps collect the very fine particles of carbon and prevents the normal filter paper from becoming clogged.]
To the slightly cooled ethanol mixture containing the lactose, add 1 mL water. Suction filter the mixture through the Celite filter pad, making sure the filtrate is clear. If the filtrate is cloudy, heat it up and
add another 0.5 mL of water. Transfer the filtrate to a 125 mL Erlenmeyer flask, heat it until it clears, then allow to cool slowly. Stopper the flask and allow it to stand in your locker until your next lab period.
Collect the crystals of lactose by suction filtration, and wash the product with a small amount of cold 95% ethanol. Thoroughly dry the lactose and determine its weight and melting point. Determine the percentage yield of lactose from the powdered milk, and show your calculations.
B. Chemical Tests for Proteins and Carbohydrates
Chemical Tests for Proteins and Amino Acids
In this experiment, you will perform chemical tests on the sample of casein which you isolated from milk, in order to determine the presence of specific amino acids in this type of protein. The tests will also be carried out on the amino acids, to help you identify a positive test with your sample, and on egg albumin, which is the main protein present in egg whites and is similar to the lactalbumin found in milk. While there are literally dozens of tests that are characteristic for only certain amino acids, we will carry out only three.
You will use the aqueous solution of casein which you prepared above (suction filter it if it is cloudy), along with stock solutions of egg albumin, tyrosine, glycine, and cysteine which have been prepared for you. Since some of the reagents used in these tests are toxic and/or corrosive, wear gloves, carry out the FIRST TWO tests in the fume hoods, and dispose of your waste in the proper labelled containers. Since we have more students than fume hoods, you will have to apportion your time carefully and stagger the amino acid tests with the carbohydrate tests, which you can carry out at the bench.
Procedures
1. Millon's Test
Millon's test is given by any compound containing a phenolic hydroxy group. Consequently, any protein containing tyrosine will give a positive test of a pink to dark-red colour. The Millon reagent is a solution of mercuric and mercurous ions in nitric and nitrous acids (CAUTION: MILLON'S REAGENT IS HIGHLY TOXIC AND HIGHLY CORROSIVE). The red colour is probably due to a mercury salt of nitrated tyrosine.
Procedure: Place 1 mL of casein, 2% egg albumin, and 0.1 M tyrosine into separate, labelled, 12 x 75 mm test tubes. Add 3 drops of Millon's reagent and immerse the tubes in a boiling water bath for 5 minutes. Cool the tubes and record the colours formed.
2. Ninhydrin Test
The ninhydrin reaction is used to detect the presence of ![]() -amino
acids and proteins containing free amino groups. When heated with ninhydrin, these
molecules give characteristic deep blue colours (or occasionally pale yellow). The
reactions involved in this test are shown below.
-amino
acids and proteins containing free amino groups. When heated with ninhydrin, these
molecules give characteristic deep blue colours (or occasionally pale yellow). The
reactions involved in this test are shown below.
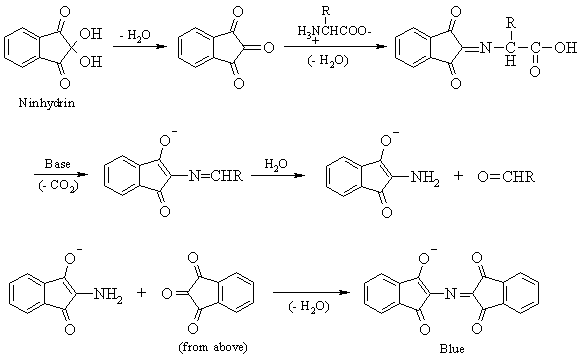
Procedure: Place 1 mL of of casein, 2% egg albumin, and 0.1 M glycine into separate, labelled, 12 x 75 mm test tubes. Add 4 drops of 0.1% ninhydrin solution. (CAUTION: NINHYDRIN IS A CARCINOGEN - AVOID DIRECT CONTACT) Add a boiling chip to each test tube and heat to boiling in a hot-water bath. Record the results.
3. Sulfur Test
The presence of sulfur-containing amino acids such as cysteine can be determined by
converting the sulfur to an inorganic sulfide through cleavage by base. When the resulting
solution is combined with lead acetate, a black precipitate of lead sulfide results.
Sulfur-containing protein ----NaOH----> S2-
----Pb2+----> PbS
Procedure: Place 1 mL of casein, 2% egg albumin, and 0.1 M cysteine into separate, labelled 16 x 150 mm test tubes. Add 2 mL of 10% aqueous sodium hydroxide. Add 5 drops of 10% lead acetate solution. Stopper the tubes and shake them, then remove the stoppers and heat in a boiling water bath for 5 minutes. Cool and record the results.
Chemical Tests for Carbohydrates
In this experiment, you will perform tests and reactions on the sample of lactose which you isolated from milk and on samples of selected other mono- and disaccharides.
1. Benedict's Test
Benedict's test determines whether a monosaccharide or disaccharide is a reducing
sugar, and is hence similar in purpose to the Tollens test. To give a positive test,
the carbohydrate must contain a hemiacetal which will hydrolyse in aqueous solution to the
aldehyde form. Benedict's reagent is an alkaline solution containing cupric ions, which
oxidize the aldehyde to a carboxylic acid. In turn, the cupric ions are reduced to cuprous
oxide, which forms a red precipitate.
RCHO + 2Cu2+ + 4OH- ----->
RCOOH + Cu2O + 2H2O
Procedure Place 15 drops of the following 1% carbohydrate solutions in separate, labelled 12X75 mL test tubes: glucose, fructose, sucrose, lactose, and maltose. Also place 1 mL of distilled water in another tube to serve as a control. To each tube, add 1 mL of Benedict's reagent and heat the tubes in a boiling water bath for 5 minutes. Remove the tubes and note and record the results.
2. Barfoed's Test
Barfoed's test is similar to Benedict's test, but determines if a carbohydrate is a
monosaccharide or a disaccharide. Barfoed's reagent reacts with monosaccharides to produce
cuprous oxide at a faster rate than disaccharides do:
RCHO + 2Cu2+ + 2H2O ----->
RCOOH + Cu2O + 4H+
Procedure: Place 15 drops of the following 1% carbohydrate solutions in separate, labelled 12X75 mL test tubes: glucose, fructose, sucrose, lactose, and maltose. To each tube, add 1 mL of Barfoed's reagent and heat the tubes in a boiling water bath for 10 minutes. Remove the tubes and note and record the results.
3. Hydrolysis Test for Glucose
Disaccharides and polysaccharides can be hydrolyzed in acidic solution into their component monosaccharides, and then submitted to chemical tests like Benedict's test. In this experiment, several disaccharides and a sample of starch will be hydrolyzed, and tested for the presence of glucose. The glucose test will be carried out using a commercially available product called Tes-Tape. Available at most drug stores, the tape contains the enzymes glucose oxidase and peroxidase, as well as ortho-toluidine. The glucose oxidase oxidizes glucose to gluconic acid and hydrogen peroxide. Once formed, the hydrogen peroxide reacts with peroxidase to produce oxygen, which oxidizes the ortho-toluidine to give green-coloured products.
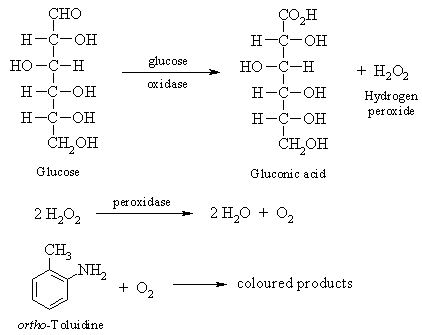
Procedure: Place 5 mL of the following 1% carbohydrate solutions in separate, labelled 16 x 150mm test tubes: sucrose, lactose, maltose, and starch. Add 3 drops of concentrated hydrochloric acid to each of the tubes, and heat them in a boiling water bath (400 mL beaker) for 10 minutes. Cool the tubes in an ice bath. Carefully neutralize each of the four solutions with 10% sodium hydroxide, using litmus or pH paper. The pH MUST be neutral or very slightly alkaline in order for the Tes-Tape to work. If necessary, make final pH adjustments with 0.1M HCl and/or 0.1M NaOH solutions. Test each solution with Tes-Tape (by placing a drop on the tape and recording the colour change - use plain distilled water as a control) and if time permits, with Benedict's reagent. Record the results and compare them with those obtained earlier with the Benedict's tests on the unhydrolysed carbohydrates.
Questions you should be able to answer
1. What component of milk is responsible for its white colour?
2. Why is lactose much more soluble in water than in ethanol?
3. Why does a disaccharide molecule not have exactly twice the molecular formula of a
monosaccharide molecule?
4. Explain what is meant by the term "reducing sugar", and what feature of a
saccharide is responsible for it being so.
5. Write a detailed reaction mechanism for the acid-catalyzed hydrolysis of lactose to
its component monosaccharides. Would the reaction work in basic solution? Why?
6. The specific rotations of ![]() - and
- and ![]() -lactose are +92.6o and + 34.2o. When
-lactose are +92.6o and + 34.2o. When ![]() -lactose is dissolved in water at room temperature, the optical
rotation of the solution changes from +92.6o to +52.3o. When ethanol
is added to the solution, pure
-lactose is dissolved in water at room temperature, the optical
rotation of the solution changes from +92.6o to +52.3o. When ethanol
is added to the solution, pure ![]() -lactose crystallizes back out
of the solution. Explain what is happening here, using detailed reaction mechanisms to
assist you in your explanation.
-lactose crystallizes back out
of the solution. Explain what is happening here, using detailed reaction mechanisms to
assist you in your explanation.
| Go to: | Instructions for Printing this Document Chem2O06 Home Page. |
29nov97; wjl