| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 2. Part A: Procedures.
Thin Layer Chromatography of Analgesic Drugs
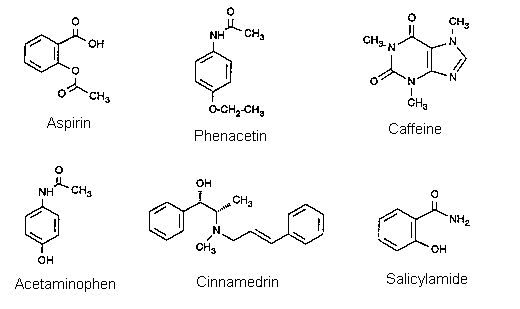
In this experiment, TLC will be used to examine the composition of various analgesic (pain relieving) drugs. The best known of these is aspirin, but several other chemically similar compounds are (or were) also used as analgesics. Among these are phenacetin, salicylamide and acetaminophen. Caffeine is sometimes added to these formulations to overcome drowsiness. A few other compounds such as Ncinnamylephedrine (cinnamedrine) and diphenylpyrilene are included for other therapeutic effects, such as antispasmodic or slight sedative action. In addition to the active ingredients, the tablets of these drugs contain starch, lactose, and other substances that act as binders and permit rapid solution, and sometimes also inorganic bases. The objective of this experiment is to identify an unknown drug tablet by a TLC comparison with standard compounds. It will take about 70 minutes to complete: do not start too late.

PROCEDURE:
The unknown will be one of those listed below:
| Drug Brand-Name | Ingredients |
| Anacin | aspirin (448 mg) caffeine | Empirin | aspirin (225 mg) phenacetin (150 mg) caffeine (30 mg) |
Excedrin | acetaminophen (320 mg) salicylamide* (128 mg) caffeine (65 mg) |
Midol | acetaminophen (455g) caffeine (45g) |
Tylenol | acetaminophen (325 mg) | Oradrine | acetaminophen (162 mg) salicylamide (227 mg) caffeine (32.4 mg) phenylpropanolamine hydrochloride (25 mg) codeine phosphate (8.1 mg) diphenyl pryaline hydrochloride (2 mg) |
The reference compounds are:
ASP - Aspirin (acetylsalicylic acid)
ACE - Acetaminophen (4-acetamidophenol)
CAF - Caffeine
PHE - Phenacetin (4-acetophenetidine)
Fine capillaries for applying spots can be made from pasteur pipets. Your demonstrator will assist you in this.
Use the chromatography jar provided with its cover. The developing solvent system is a mixture of:
120 mL toluene
60 mL ether
18 mL acetic acid
1 mL methanol
Place 5 - 6 mL of this solvent mixture (which is provided to you) in the chromatography jar, cover tightly and allow to stand 5 - 10 minutes before using. CHROMATOGRAPHY JARS SHOULD BE FILLED IN THE HOOD. THE CHROMATOGRAMS MUST BE DEVELOPED AND DRIED IN THE HOOD.
Crush one tablet of the unknown sample into a powder and dissolve in 4 mL of methanoltoluene (1:1) in a 4" test tube. The tablets all weigh approximately 0.5 g. The reference standards have been prepared at a strength of 25 mg/mL. [NOTE: some insoluble material will not dissolve. This is normal.]
Use the commercially prepared fluorescent Silica Gel TLC sheet, supported on plastic sheet, 8.5 x 3.5 cm. These sheets have been activated by heating at 120E for 20 minutes.
You will have five solutions (4 reference compounds and 1 unknown) to examine. You should use two plates. They should be spotted on the coated side in a line 1 cm from one end of the sheet, equally spaced apart, with the outer two spots about 0.75 cm from the edge of the sheet. The unknown should be placed in the centre, with one reference compound on each side. You should use one plate to run your unknown plus two of the reference samples, and the other plate to run the unknown plus the remaining two reference samples.
To apply a sample, touch the rounded end of a melting point tube to the solution, and then gently touch the Silica Gel plate at the proper spot. Use a fresh melting point tube for each sample. The sample spots should not be larger than 2 - 3 mm diameter.
When each plate has been prepared, place the plate, spotted end down, in the developing jar. Make sure that the solvent pool begins below the spots. Cover tightly, do not disturb, and allow about 5 - 10 minutes for the solvent to rise to within about 1 cm from the top of the sheet - do not allow the solvent to run all the way to the top of the plate! Remove the sheet and immediately mark the solvent front. Allow the sheet to dry.
The colourless compounds are visualized by illumination of the plate with an ultraviolet lamp. Many substances, particularly aromatic compounds, will show a bright fluorescence, which may have a characteristic colour. The thin layer plates used contain a trace of fluorescent dye. Compounds which are fluorescent show up as bright spots on a light background; any others appear as a dark spot since they quench the fluorescence of the background dye. Circle the spots lightly in pencil, and note any distinctive colours.
(a) Calculate the Rf values of the reference compounds and the components of the unknown.
(b) Draw the chromatogram to scale in your lab book; identify and label the spots in the chromatogram, including as many of the spots in the unknown as possible.
(c) From the number, position and appearance of the spots in the unknown, and the composition of the possible unknowns, identify your unknown analgesic.
| Go to: | Instructions for Printing this Document Experiment 2 - Main Page Introduction Part B Procedures Chem2O06 Home Page. |
16sep97; wjl