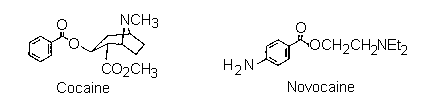
| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 9. Reactions of Carboxylic Acids & Their Derivatives.
Students should begin the benzocaine preparation as quickly as possible and use the 2 hr reflux period to carry out the test experiments.
References: Ege, Chapter 14,15
This experiment is in two parts. The first is a Preparation in which you convert a carboxylic acid to one of its derivatives, an ester. The second part is consists of a couple of simple testtube reactions that illustrate some of the properties of these various classes of compounds. Don't forget to write equations for all reactions you observe.
A. Synthesis of Benzocaine
Background
The scientific work that led to the discovery of the local anaesthetic "benzocaine", is typical of how many biologically active synthetic drugs have been developed from naturally occurring substances. For centuries the Amerindians of the Andes have been using the leaves of the 'Coco bush' (Erythroxylon coca) as a mild stimulant. They would (and still do) chew the leaves to help them cope with the exigencies of life in a harsh environment altitude, hunger, inclement weather, and a precipitous terrain make several demands on endurance. In due course, chemists investigated the components of the coco leaves, and finally the active component, the alkaloid "cocaine", was isolated pure in 1862. In the following years its properties were investigated, and by the 1880's it was in use as an anaesthetic in surgery and dentistry, one of the first such drugs to alleviate pain in medical procedures.
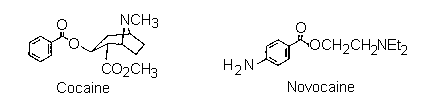
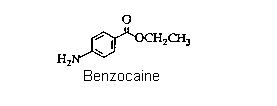
However, it was quickly realized that cocaine also had two major disadvantages, first, the lethal dose was not a great deal more than the therapeutic dose, so safe administration of the drug in surgery was unreliable. Secondly, cocaine causes serious additional problems (as our society knows to its cost) as well as potentially irreversible damage to the central nervous system. Consequently, as soon as the structure of cocaine was established (in 1910), chemists began preparing analogues, in the hope of finding one that would have lower toxicity but equivalent efficiency. This search was based on the assumption that certain structural features were important for activity. As the list of successful analogues grew, it became apparent what these were: that there should be an aromatic acid derivative, with the carbonyl group separated from a tertiary amine nitrogen by a chain of two to four atoms. An example is Novocain, which is one of the most successful synthetic drugs in this family in use. A simplier analogue is the ethyl ester of paraaminobenzoic acid, called "Benzocaine". Clearly this lacks the tertiary amine component, so not surprisingly, it is less active as an injectable drug. Nevertheless, it retains anaesthetic properties, and is used extensively as a topical pain reliever, for example in medications for treating sunburn. This is the target of the synthesis part of this experiment.
A similar story of discovery, investigation, and exploration lies behind the development of the synthetic antimalerial "Chloroquin" from naturally occurring quinine, and of synthetic insecticides like "Deltamethrin" from naturally occurring pyrethrins.
Procedure
Place 2.7 g of paminobenzoic acid in a 250 mL roundbottomed flask and add 40 mL of 95% ethanol, swirling gently to help dissolve the solid (not all the solid will dissolve). Cool the mixture in an ice bath and slowly add 2.5 mL of concentrated sulfuric acid (CAREFULLY!). A large amount of precipitate will form when the sulfuric acid is added, but this solid will slowly dissolve during the reflux that follows. Attach a reflux condenser and heat the mixture under reflux on a heating mantle for 75 min. Take care not to overheat the flask and have too violent a reflux rate. (Again, ensure that your heating mantle is supported on an iron ring above the level of the desk.) Stir the contents of the flask with a magnetic stirbar or by swirling at approximately 15 minute intervals during the first hour of the reflux.
After the 75 min reflux period remove the heating mantle and allow the flask to cool (you may use a beaker of cold water if you wish to hasten cooling). When the flask is cold, transfer the contents to a 250 mL beaker and slowly add small portions of a 10% sodium carbonate solution (about 35 mL needed) to neutralize the mixture. After each addition of the sodium carbonate solution, extensive gas evolution (frothing) will be perceptible until the mixture is nearly neutralized. When gas no longer evolves as you add a portion of sodium carbonate, check the pH of the solution and add further portions of sodium carbonate until the pH is 9 or above.
Add 70 mL of ether to the beaker and stir with a glass rod to dissolve as much of the solid benzocaine as possible - do not worry if all the solid does not dissolve. Pour the mixture through a glass funnel (residual solids included) into a separatory funnel (250 mL or larger) and rinse your beaker with a few mL of additional ether to ensure all the benzocaine has been transferred to the separatory funnel. Shake the separatory funnel 3 - 4 times, releasing the ether vapour pressure after each shaking. Allow the mixture to separate and save the upper ether layer. Dry the ether layer with sodium sulfate (about 2 spatulas), gravityfilter the ether layer into an Erlenmeyer flask to remove the drying agent, and remove the ether and ethanol by evaporating them on a steam bath in a HOOD (CARE! no flames) until all of the ether (bp 35oC) has been removed. About 5 to 8 mL of material (likely in two phases) should remain; some will be residual ethanol and the rest, the yellowish oily material, your ester product.
Add a little hot 95% ethanol and heat the mixture on the steam bath until all the oil dissolves. Add water (dropwise!) to the alcohol solution until appreciable cloudiness again just appears and then cool the mixture, with VIGOROUS swirling, in an ice bath. Collect the benzocaine by vacuum filtration, using a Buchner funnel. Allow the solid to dry at room temperature on a weighed piece of filter paper, weigh it, calculate the percentage yield, and determine its melting point. The melting point of pure benzocaine is 92oC. Place your sample in the container provided.
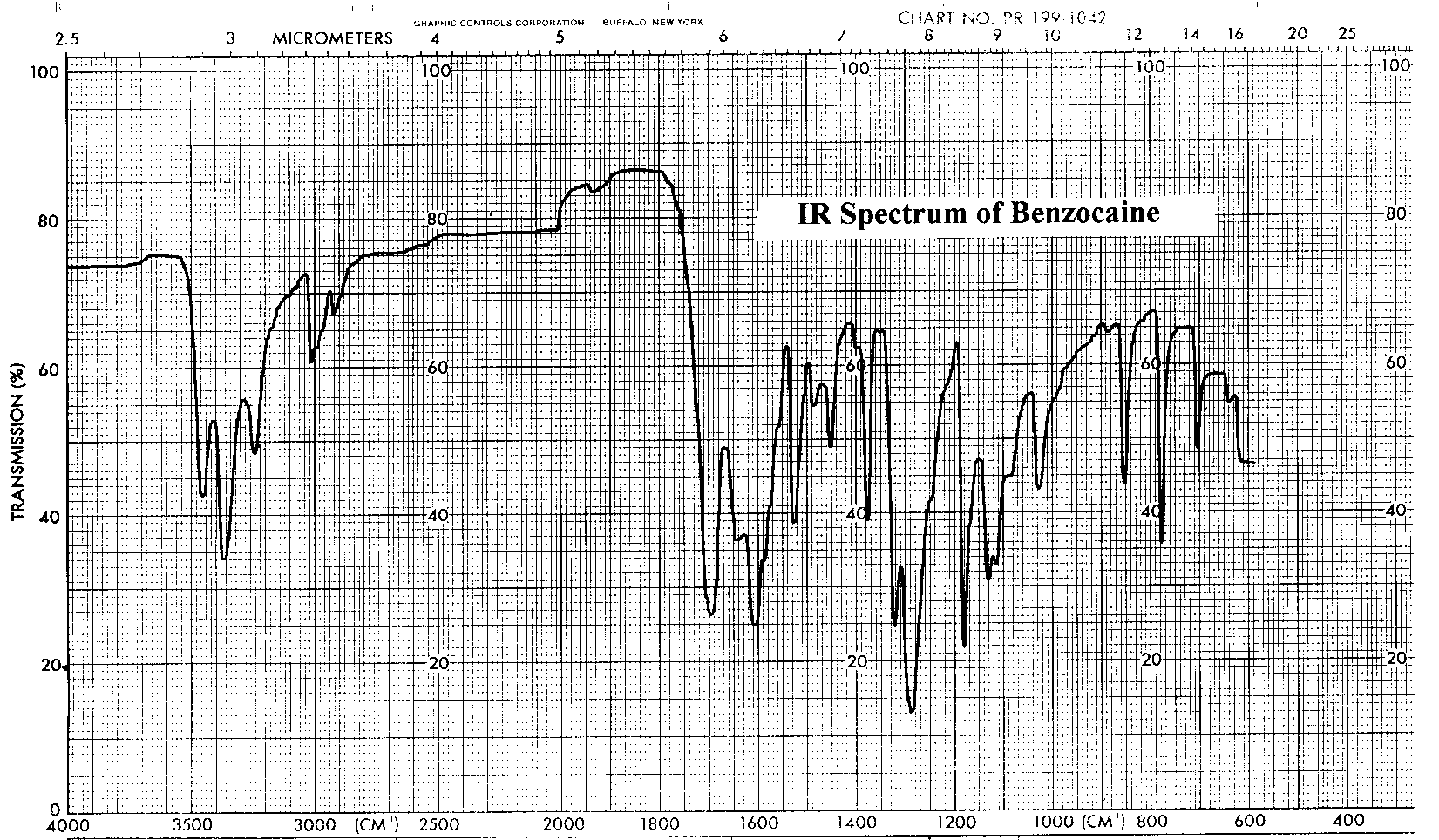
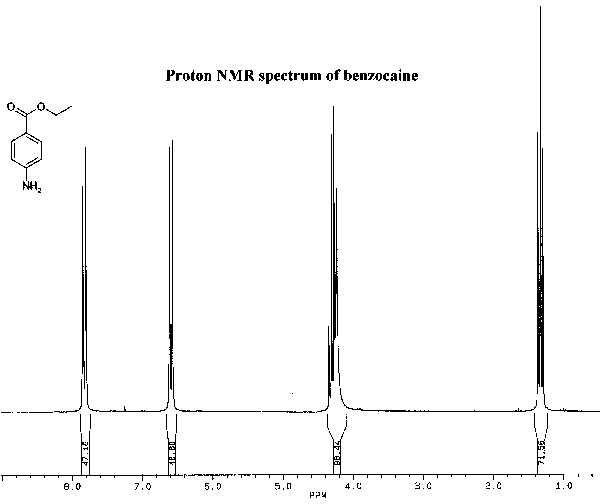
The infrared and proton NMR spectra of benzocaine are shown below. Assign all significant peaks in both spectra and include them in your report.
B. Carboxylic Acid Salts and Derivatives
(a) Salt Formation Carboxylic acids may be neutralized by strong bases to give salts. Acids which may be only slightly soluble in water can be extracted from their solutions in an organic solvent by aqueous base, as the salts are usually watersoluble: RCOOH + NaOH ---> RCOO-Na+ + H2O.
Procedure: In each of two test tubes, place 0.1 g of benzoic acid. To one tube add 3 mL of cold water, to the other add 3 mL of 10% sodium hydroxide solution. Shake both tubes, observe, and record the results. Then acidify salt with diluted hydrochloric acid.
(b) Salt Hydrolysis Most carboxylic acids are only partially dissociated in aqueous solution. Consequently, when the salt of a carboxylic acid is dissolved in water, it is partially hydrolyzed to the undissociated acid and hydroxide ion: RCO-Na+ + H2O ---> RCOOH + NaOH. This hydrolysis can readily be demonstrated by testing the solution of a carboxylic acid salt with an indicator such as litmus paper.
Procedure: Dissolve approximately 0.2 g of sodium acetate in 5 mL of distilled water, test the resulting solution with litmus paper, and note the result.
(c) Acid Chlorides [These experiments MUST BE DONE IN THE HOOD.] Add drop by drop 1 mL of acetyl chloride to 1 mL of butanol. Allow to stand for about 2 minutes and then pour it carefully into 5 mL of water (acetyl chloride reacts vigorously with water). Note the odour. Remove a little of the insoluble layer with a dropper and test its solubility in 5% NaOH solution. Explain the results with equations.
(d) Amides Dissolve a little acetamide in water and determine the pH of the solution using pH-paper. Compare your results with that for (i) aniline in water, and (ii) ethylamine in water. Add 0.5g acetamide to 5 mL of 10% sodium hydroxide solution in a test tube, and gently warm the solution: test any vapour evolved with moist pH paper (do not let the pH paper touch the walls of the test tube).
Questions you should be able to answer
1. Write an equation which accounts for the solubility of benzoic acid in aqueous base.
2. Write an equation to illustrate what happens in the reaction of acetamide with 10% sodium hydroxide solution.
3. Use your observations in Part B to explain how a waterinsoluble organic acid might be freed of non acidic impurities.
4. Was the aqueous solution of sodium acetate acidic or alkaline? Explain why, with the help of an equation.
5. Acetamide (MW = 59.07) has a melting point of 79-81oC and is soluble in water, whilst ethyl acetate (MW = 88.11) boils at 76.5-77.5oC and is insoluble in water. Explain.
6. In B part (d), why is it important not to touch the walls of the tube with pH paper?
7. In part A, what is the solid that precipitates on adding sulphuric acid?
8. In Novocaine, which nitrogen would be protonated first? Why?
| Go to: | Instructions for Printing this Document Chem2O06 Home Page. |
29nov97; wjl