| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Microscale Laboratory Techniques - Handling of Liquids
Since one rarely works with volumes larger than 2-3 mL, graduated cylinders are rarely used in microscale experiments. Instead, one uses smaller scale volumetric devices such as syringes, automatic pipets, and calibrated disposable Pasteur pipets.
Automatic Pipets are commonly used in microscale organic and biochemistry laboratories. They are available in different sizes, and can deliver accurate volumes of aqueous solutions from 0.10 mL to 1.0 mL. They were not designed for use with organic solvents and are generally less accurate depending on the liquid. Automatic pipets are very expensive, and it is critical that the student handle them carefully and responsibly. Follow the steps outlined below to use an automatic pipet:
1. Select the desired volume by adjusting the micrometer control on
the pipet handle.
2. Place a plastic tip on the pipet. Be certain that the tip is attached
securely.
3. Push the plunger down to the first detent position. Do not press the
plunger to the second position. (If the plunger is pressed to the second detent, an
incorrect volume of liquid will be delivered).
4. Dip the tip of the pipet into the liquid sample. Do not immerse the
entire length of the plastic tip in the liquid. It is best to dip the tip only to a depth
of about one centimeter.
5. Release the plunger slowly. Do not allow the plunger
to snap back, or liquid may splash up into the plunger mechanism and ruin the pipet.
Furthermore, rapid release of the plunger may cause air bubbles to be drawn into the
pipet. At this point the pipet has been filled.
6. Move the pipet to the receiving vessel. Touch the tip of the pipet to
an interior wall of the container, and slowly push the plunger down to the first detent.
This action will dispense the liquid into the container.
7. Pause one or two seconds and then depress the plunger to its second
detent position to expel the last drop of liquid. The action of the plunger may be stiffer
in this range than it was up to the first detent.
8. Withdraw the pipet from the receiver. If the pipet is to be used with
a different liquid, remove the pipet tip and discard it.
Syringes are especially useful when anhydrous conditions must be maintained during an experiment. The needle can be inserted through a rubber septum sealing the reaction vessel, and the liquid added to the reaction mixture. We use plastic (polyethylene) syringes which, although they are called "disposable", can be cleaned and re-used. While the polyethylene barrels are impervious to most solvents, the plungers are made of a less inert material; thus, these syringes cannot be used with __________. To fill the syringe, insert the needle into the liquid and draw in the required volume. Withdraw the syringe and pull the barrel back ever so slightly to draw any liquid remaining in the needle into the syringe.
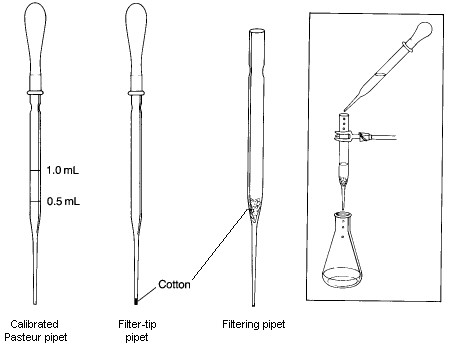
Disposable Pasteur pipets are used for dispensing small quantities of liquids, as filtration devices, and as columns for small-scale column chromatography. Although they are considered disposable, you should be able to clean them for reuse as long as the tip remains unchipped.
Pasteur pipets may be calibrated for use in operations where the volume does not need to be known precisely, such as for measurement of solvents need for extraction and for washing a solid obtained following crystallization. To calibrate a Pasteur pipet, weigh 0.5 g (0.5 mL) of water into a small test tube on a balance. Attach a rubber bulb to a short Pasteur pipet. Squeeze the rubber bulb before inserting the tip of the pipet into the water. Try to control how much you depress the bulb so that, when the pipet is placed into the water and the bulb is completely released, only the desired amount of liquid is drawn into the pipet. When the water has been drawn up, place a mark with an indelible marking pen at the position of the meniscus. A more durable mark can be made by scoring the pipet with a file. Repeat this procedure with 1.0 g of water, and make a 1-mL mark on the same pipet.

A Filtering Pipet is used to remove solid impurities from a liquid with a volume less than 10-mL. To prepare it, a small piece of cotton is inserted into the top of a Pasteur pipet and pushed down to the beginning of the lower constriction in the pipet. It is important that enough cotton is used to collect all the solid being filtered; however, the amount used should not be so large that the flow rate throught the pipet is significantly restricted. The cotton plug can be pushed down with a long thin object such as a glass stirring rod or a wooden applicator stick. In some cases, such as when filtering a strongly acidic mixture or when performing a very rapid filtration, it may be better to use glass wool in place of the cotton, even though it is not quite as good as a filtering aid. To conduct a filtration, the filtering pipet is clamped so that the filtrate will drain into an appropriate container. The mixture to be filtered is transferred to the filtering pipet with another Pasteur pipet. If the volume of the mixture being filtered is less than 1-2 mL, you should rinse the filter and plug with a small amount of solvent after the last of the filtrate has passed through the filter. If desired, the rate of filtration can be increased by genly applying pressure to the top of the pipet using a pipet bulb.
A Filter-tip Pipet is useful for transferring volatile solvents during extractions and in filtering very small amounts of solid impurities from solutions. It is made by loosely shaping a tiny piece of cotton into a ball, and pushing it to the bottom of the pipet using a wire with a diameter slightly smaller than the inside diameter of the narrow end of the pipet. If it is difficult to push the cotton into the tip, you've probably used too much cotton. To use the filter-tip pipet, simply draw the mixture to be filtered into the pipet using a pipet bulb and then expelling it. With this procedure, small amounts of solid will be captured by the cotton.
| Go to: | Instructions for
Printing this Document |
wjl; 25-nov-97