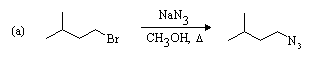
Good leaving group on 1o carbon, strong weakly basic nucleophile, and polar weakly ionizing solvent favour SN2 substitution.
| Assignment #2 | ANSWERS | November 13, 1996 |
1. Predict the principal products of the following reactions, showing stereochemistry where appropriate.
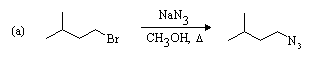
Good leaving group on 1o carbon, strong weakly basic nucleophile, and polar
weakly ionizing solvent favour SN2 substitution.
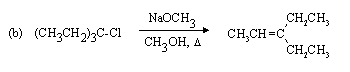
Strongly basic nucleophile, heat, and polar weakly ionizing solvent favour E2 elimination.
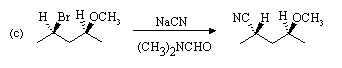
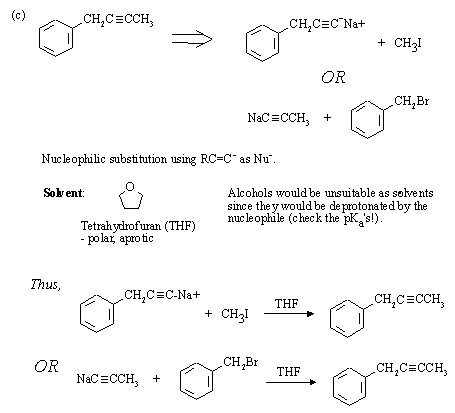
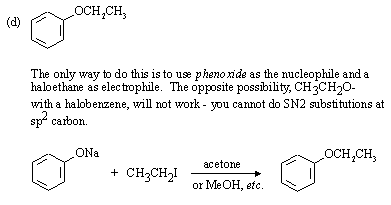
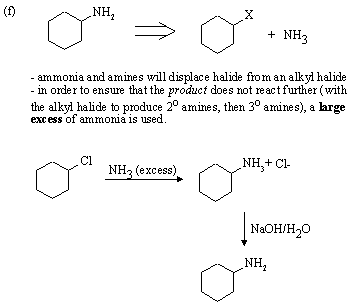
Strong, weakly basic nucleophile, and polar aprotic solvent favour SN2 substitution.
Bromide is a good leaving group; methoxide (CH3O-) is not. Note
inversion of configuration.
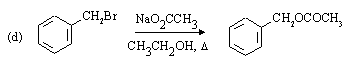
1o benzylic carbon, weakly ionizing solvent favour SN2 substitution.
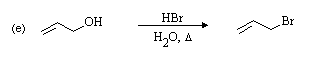
1o allylic carbon, acidic conditions (allowing protonation
of OH), and strong nucleophile favour SN2 substitution. SN1 substitution
might also be expected because of the strongly ionizing conditions, but the result is the same.
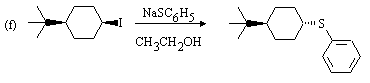
2o carbon bearing excellent leaving group, weakly ionizing solvent, and strong weakly basic
nucleophile favour SN2 substitution.
2. Suggest a synthesis of (R)-2-butanol from (R)-2-bromobutane. Two steps may be required.
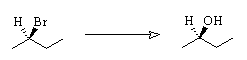
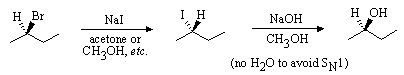
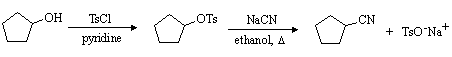
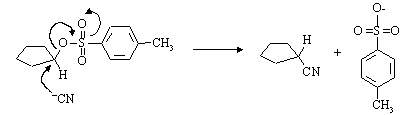
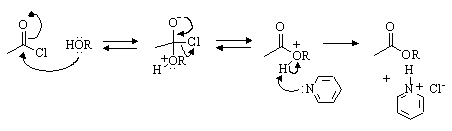
(a) Write a mechanism for the nucleophilic substitution reaction in the equation above.
(b) Carboxylic acid esters are synthesized by a similar procedure to that used for the synthesis of sulfonate esters. For example,
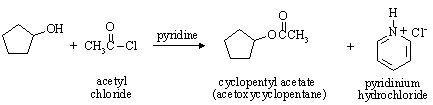
Write a reasonable mechanism for the formation of cyclopentyl acetate from cyclopentanol and acetyl chloride, based on your knowledge of sulfonate ester formation.
(c) What would be the product of reaction of (1R,2S)-2-methylcyclopentanol with acetyl chloride in pyridine? (Give the structure and name)
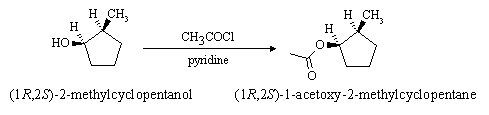
(d) While tosylate and mesylate are very good leaving groups in nucleophilic substitution reactions, acetate and other carboxylate anions are not. Explain why.
Tosylate and mesylate have pKa < 0; they are good leaving groups because they are very weak bases. Acetic acid has pKa 4.8; acetate (CH3CO2-) is therefore a stronger base than TsO- or MsO- and is thus a poorer leaving group.
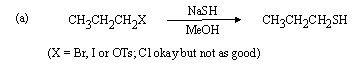
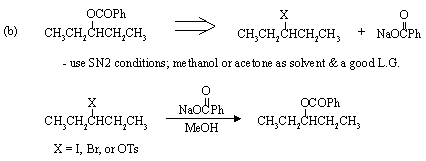
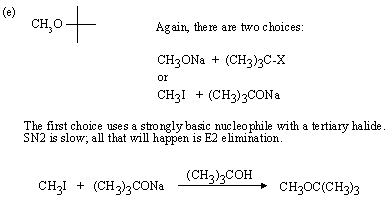
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
01nov96; wjl