| Assignment #3 | ANSWERS | January 9, 1997 |
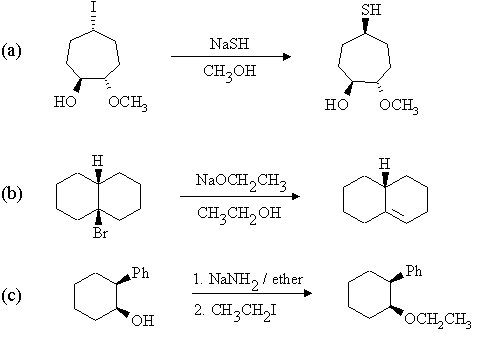
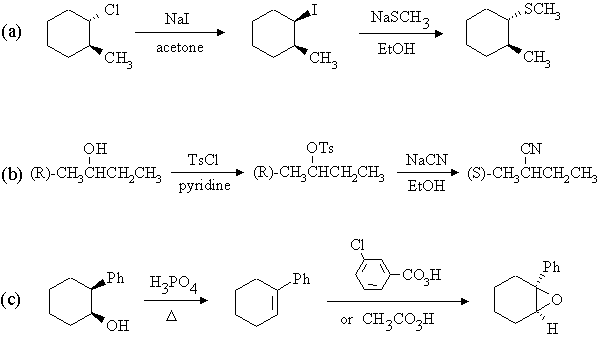
1. Predict the principal products of the following reactions, showing stereochemistry where appropriate.



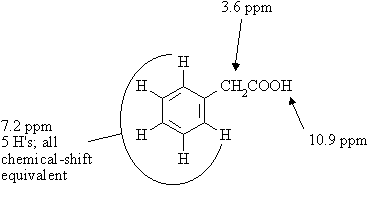
Answer:
The molecular formula indicates 5 sites of unsaturation; with this many, the molecule is very likely to be aromatic.
The IR spectrum shows a strong peak at ~1700 cm-1, indicative of a carbonyl, and a broad absorption in the 2500-3500 cm-1 range indicative of an OH group. Thus, the molecule is either a carboxylic acid or a hydroxyketone (i.e. a ketone that also contains an alcoholic OH group), hydroxyaldehyde, or hydroxyester.
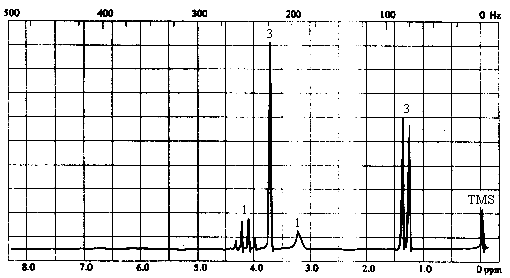
The 1H resonance at 10.9 ppm in the NMR spectrum is indicative of a carboxylic acid (COOH) or an aldehyde (CHO). If it's an aldehyde, then there should also be an OH proton elsewhere in the spectrum in order to be compatible with the IR spectrum. There isn't, so we can conclude that the molecule is a carboxylic acid. The 5H singlet at 7.2 ppm is due to aromatic protons; the compound is a monosubstituted benzene derivative. The COOH group must be part of an alkyl chain. So far, we've accounted for C6H5 and COOH, leaving just a CH2 unaccounted for. The CH2 protons appear at 3.7 ppm. The only way that these three fragments can be put together is as C6H5CH2COOH. The structure and assignments are:
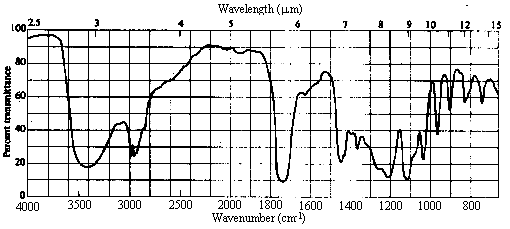
Answer:
The molecular formula indicates there to be one site of unsaturation.
The IR spectrum shows a strong peak at ~1720 cm-1, indicative of a carbonyl, and a broad absorption in the 2500-3700 cm-1 range indicative of an OH group. Again, the molecule is either a carboxylic acid or a hydroxyketone (i.e. a ketone that also contains an alcoholic OH group), hydroxyaldehyde, or hydroxyester. Note that the single site of unsaturation is now accounted for.
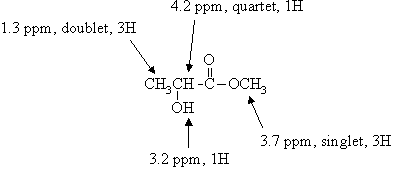
This time, the NMR spectrum shows no resonance in the 10-12 ppm range. Thus, the molecule must be either a hydroxyketone or hydroxyester. The OH proton shows up as the broad 1H resonance at 3.2 ppm. The 3H singlet at 3.7 can only be due to a methyl group attached directly to oxygen: OCH3. The 1H quartet at 4.2 ppm and the 3H doublet at 1.3 ppm are due to a CHCH3 group.
From the IR and NMR spectra, we have concluded that the molecule contains C=O, OH, OCH3, and CHCH3. The entire molecular formula is accounted for. Given that the molecule is not a carboxylic acid, there is only one way of putting these fragments together:
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
01nov96; wjl