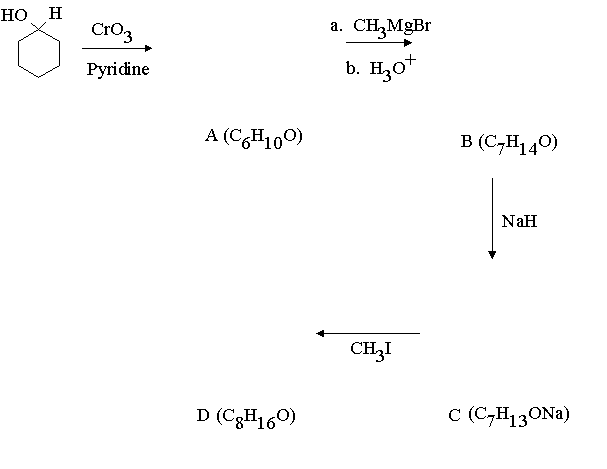
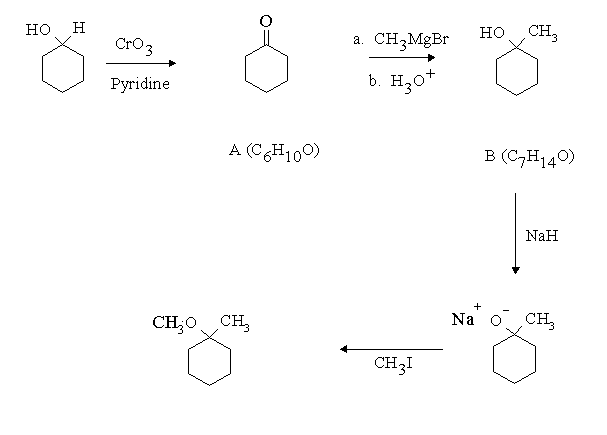
| Assignment #4 | Answers | Due: February 21, 1997 |
1.Assign structures to the intermediary compounds shown in the following reaction scheme.

Answer:
a) CH3CH(OH)CH2CH2CH3
b)
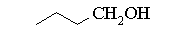
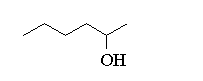
c)(CH3CH2CH2)2CHOH
d)
Answers:
a)
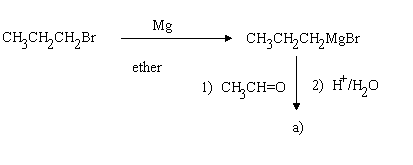
b)
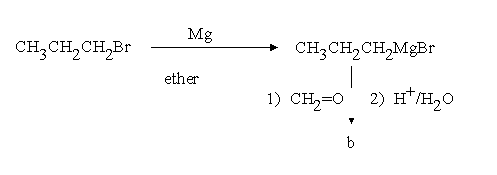
c)
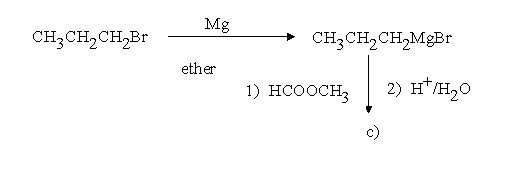
d)
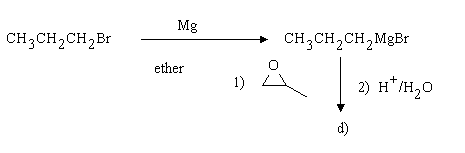
Answers:
The key to this problem is realize that acid catalysed additions to a double bond involve the formation of the most stable carbenium ion. The most stable carbenium which can be formed from acrolein is when the proton adds to the oxygen atom.
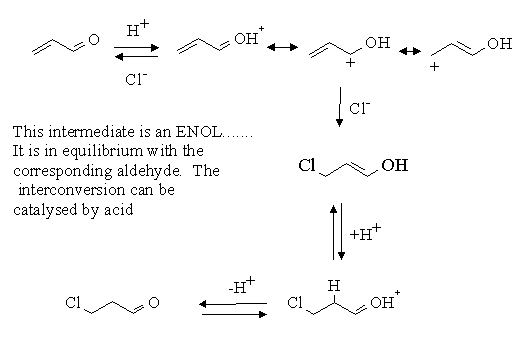
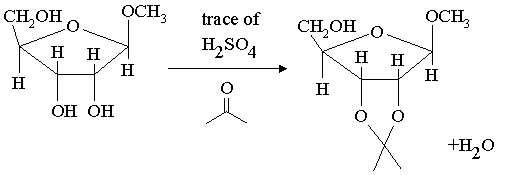
Answers:
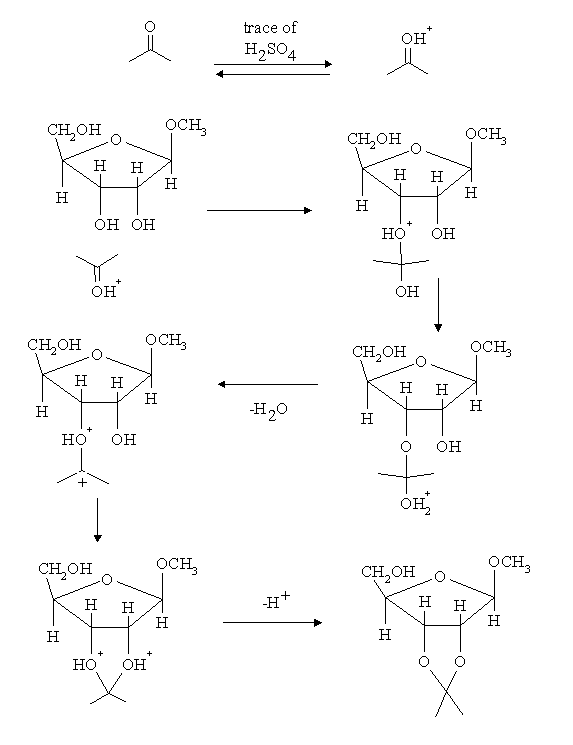
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
07 feb 97; rfc/jp