| Problem Set #11 | ANSWERS #11 | January 17, 1997 |
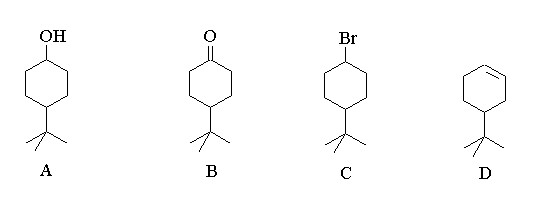
1. Show how you could prepare each of compounds B, C, and D from the starting material A.

Answers:
B could be made from A by an oxidation using pyridinium chromyl chloride or simply dichromate.
C could be made from A by treatment with HBr. Alternatively A could be converted to a tosylate and then subjected to a nucleophilic displacement using sodium bromide.
D involves an elimination of water from A. This could be accomplished directly from A by warming it in a strong sulphuric acid solution. A more gentle route would be to eliminate HBr from C using a non-nucleophilic base such as tertiary butoxide.
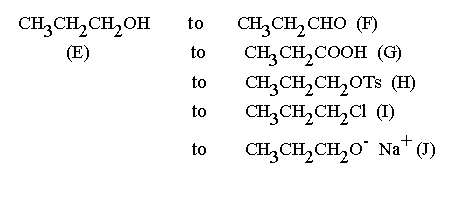
Answers:
F can be prepared from E by oxidation under anhydrous conditions using pyridinium chromyl chloride. (Watch out for oxidation to the acid.)
G involves the complete oxidation of E. Dichromate in aqueous sulphuric acid would work well.
H can be formed from E by reaction of tosyl chloride (p-toluenesulphonyl chloride) in a tertiary amine as solvent and acid acceptor. Pyridine is the usual solvent but other tertiary amines will work.
I use thionyl chloride
J can be formed by reaction of anhydrous E with sodium metal or sodium hydride.
 (K)
(K)
Answers:
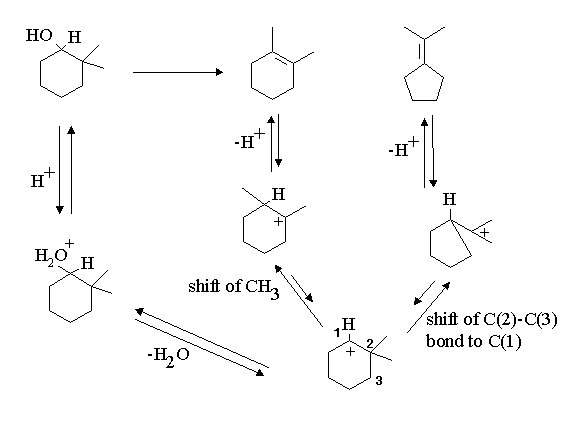
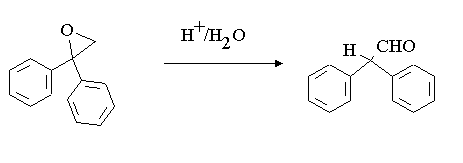
Note there is an intermediate product formed, what is this product?
Answers:
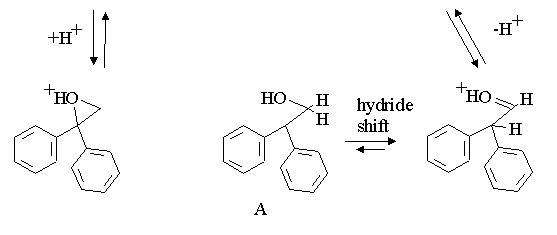
three intermediate carbocations in the reaction; note that the driving force for the hydride shift is the formation of the very stable oxygen substituted carbocation
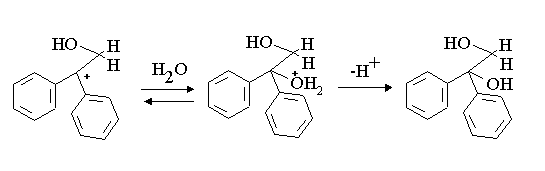
Note a competing reaction of A is collapse with water in the normal manner of the hydration of an oxirane. This gives the diol B. Note that the diol B can react with a proton and go back two steps to A and thence to the more stable aldhyde product seen.
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
24 jan 97; rfc/jp