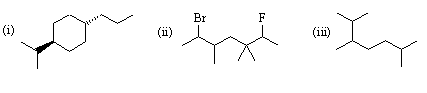
| Problem Set #4 | ANSWERS | October 17, 1997 |
1. Give IUPAC names for the following structures:
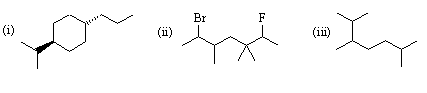
Answer:
(i) trans-4-(1-methylethyl)-1-propylcyclohexane
(ii) 6-bromo-2-fluoro-3,3,5-trimethylheptane
(iii) 2,3,6-trimethylheptane
(a) 1-chloro-2,2-dimethylpentane
(b) 3-(1,1-dimethylethyl)hexane
(c) 4-propylheptane
(d) 2-bromo-3-chloro-5-(2-methylpropyl)dodecane
Answer:
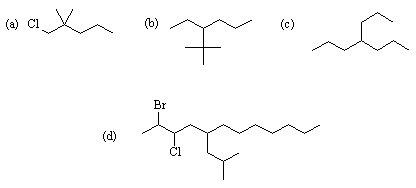
If a gauche OH-OH interaction is worth 1.6 kJ/mol, a gauche CH3-CH3 interaction 3.5 kJ/mol, and a gauche CH3-OH interaction 2.0 kJ/mol, how much more stable is your selected conformation above than the least stable conformer?
Answer:
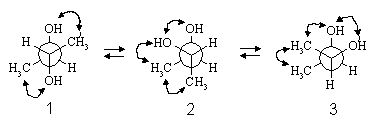
The double-headed arrows indicate gauche interactions.
Conformer 1 is the most stable because there are only two (CH3-OH) gauche interactions present, whereas there are three gauche interactions present in conformers 2 and 3 (one CH3-CH3, one CH3-OH, and one OH-OH). Conformer 1 will be 3.2 kJ/mol more stable than 2 and 3 because conformer 1 has 2 X (CH3-OH) = 2 X 2.0 = 4.0 kJ/mol worth of gauche interactions, whereas 2 and 3 have (CH3-CH3) + (CH3-OH) + (OH-OH) = 3.6 + 2.0 + 1.6 = 7.2 kJ/mol.
Invert the chair into the alternate chair. What is the orientation of the two C-Br bonds now? Identify any 1,3-diaxial interactions present in both conformers.
Answer:
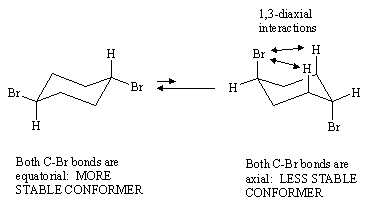
Answer:
Keq = exp(-![]() Go/RT)
Go/RT)
= exp(-7.4/{(.00831)(298)})
= exp(-2.99)
= 0.0504
Keq = [axial]/[equatorial] = 0.0504
[equatorial] = 1 / 1.0504 = 0.95
95% of ethylcyclohexane molecules are in the equatorial conformation at 25oC.
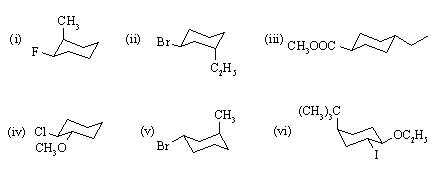
Answer:
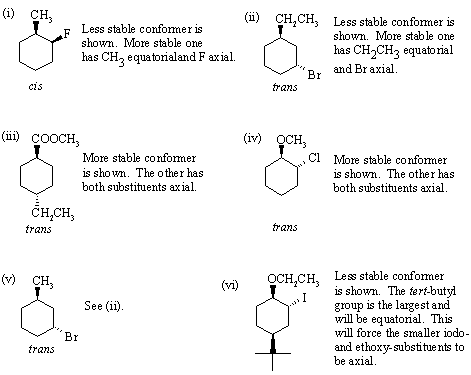
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
17oct97; wjl