Chem2O6 - 1997/98
| Problem Set #5 |
ANSWERS |
October 24, 1997 |
1. As you discover in part (c), there are two conformers in which both the A and B rings in this molecule
are chairs. They are shown below, along with the answers to (a) and (b) given for each.
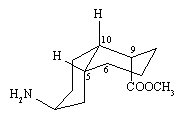 |
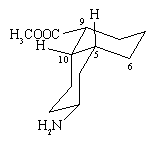 |
(a) With respect to the A-ring,
(i) the NH2 is axial
(ii) H-5 is axial
(iii) H-10 is equatorial
(iv) bond 9-10 is axial
(v) bond 5-6 is equatorial
(b) With respect to the B-ring,
(i) the COOCH3 is axial
(ii) H-5 is equatorial
(iii) H-10 is axial |
(a) With respect to the A-ring,
(i) the NH2 is equatorial
(ii) H-5 is equatorial
(iii) H-10 is axial
(iv) bond 9-10 is equatorial
(v) bond 5-6 is axial
(b) With respect to the B-ring,
(i) the COOCH3 is equatorial
(ii) H-5 is axial
(iii) H-10 is equatorial |
Since the structure on the right has the NH2 and COOCH3 groups equatorial
rather than axial, it is the more stable conformer.
2.
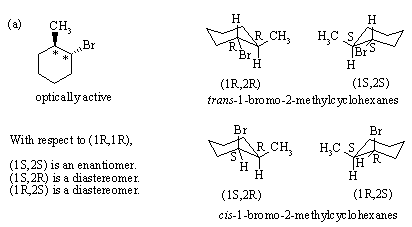
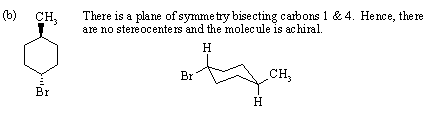
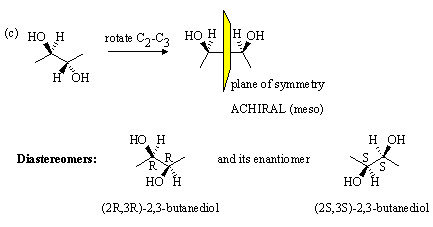
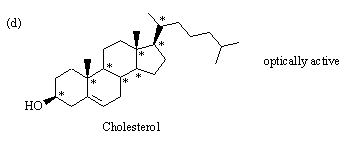
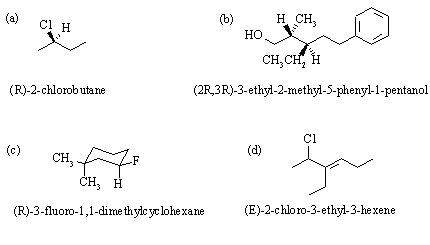
3.
4. (-)-3-chloropentanoic acid, 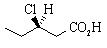 , has [
, has [ ]
D25 -27.8o in water.
]
D25 -27.8o in water.
(a) What would be the observed optical rotation of a sample of 2.5 g of the compound
in 100 mL of water, measured in a 40-cm tube?
Answer:
Since [ ]D25 =
]D25 =
 / lc,
/ lc,
the observed optical rotation  =
[
=
[ ]D25 X (lc)
= (-27.8)(0.025)(4) = -2.78o
]D25 X (lc)
= (-27.8)(0.025)(4) = -2.78o
(b) A sample of 3-chloropentanoic acid having [ ]
D25 +12.7o was recovered by resolution of a racemic mixture
of the acid. What is the enantiomeric excess of the sample recovered from the racemic mixture?
]
D25 +12.7o was recovered by resolution of a racemic mixture
of the acid. What is the enantiomeric excess of the sample recovered from the racemic mixture?
Answer:
ee = ( / [
/ [ ]D25) X 100%
]D25) X 100%
= 45.6% of (+)-3-chloropentanoic acid
24oct97; wjl







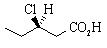 , has [
, has [