| Problem Set #6 | ANSWERS | November 1, 1997 |
1. Draw and name all the stereoisomers that are possible for each of the following compounds. Identify the enantiomers and diastereomers.

Answers:
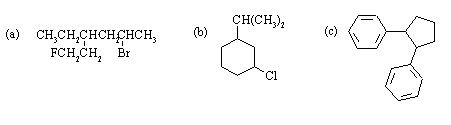
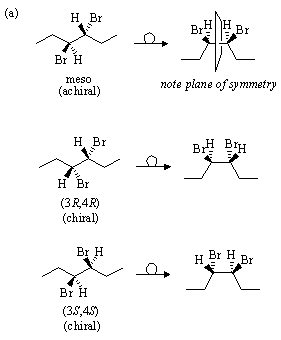
(a) There are two stereocenters in 5-bromo-3-ethyl-1-fluorohexane, and hence a maximum of four stereoisomers. The group priorities are:
At C3. 1, -CH2CH(Br)CH3; 2, -CH2CH2F; 3, -CH2CH3; 4, -H.
At C5. 1, -Br; 2, -CH2CH(CH2CH3)(CH2CH2F); 3, -CH3; 4, -H.

There are thus two diastereomeric pairs of enantiomers.
(b) There are two stereocenters in 1-chloro-3-(1-methylethyl)cyclohexane, and hence a maximum of four stereoisomers. Ignoring the H's (they are always priority 4), the group priorities for the two stereocenters are:
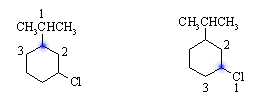
There are two enantiomeric cis-compounds, and two enantiomeric trans-compounds:

(c) Again, there are two stereocenters in 1,2-diphenylcyclopentane, and a maximum of four stereoisomers. However, in this case the two stereocenters have identical substituents; one diastereomer (the cis-compound) will have a plane of symmetry and thus be achiral. In all, there are three stereoisomers: enantiomeric trans-compounds and the cis-compound (a meso form).


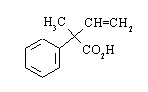
(a) Draw perspective formulas of the two compounds and identify their relationship to one another.
Answer:
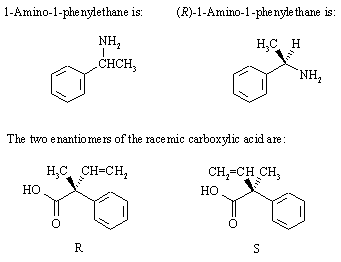
Carboxylic acids (RCO2H) form salts with amines (R'NH2) of the type RCO2-NH3+R'. In this case, reaction of the chiral amine with the two enantiomers of the carboxylic acid yields:
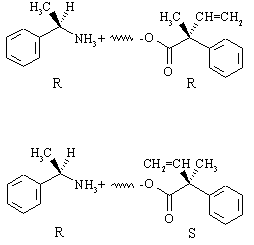
The two salts are chiral, but they are not enantiomers - they are diastereomers of one another, and thus have different physical properties and solubilities in ethanol. The compound that crystallizes out of solution (assume that it's the R,R salt) is the less soluble of the two. The other (the R,S salt) remains in solution and is isolated by simply evaporating the solvent. Overall, the two enantiomers of the carboxylic acid have been converted to two different compounds that can now be separated simply by recrystallization!
You should make molecular models of the two compounds to convince yourself of this (just use coloured balls to represent phenyl rings, to keep it simple).
(b) The two compounds were each dissolved separately in water, the solutions were made basic with 1 M aqueous sodium hydroxide in separatory funnels, and then extracted with ether. For each one, identify which compound would be present in the aqueous layer and in the ether layer.
Answer: Carboxylic acid salts are soluble in water, dissociating to the constituent ions RCO2- and R'NH3+. When hydroxide is added, the ammonium ion is deprotonated, converting it to the neutral amine which is insoluble in water. Thus, after addition of hydroxide and extraction, the amine is present in the ether layer while the carboxylate salt remains in the aqueous phase. The procedure is illustrated below for the R,R salt:
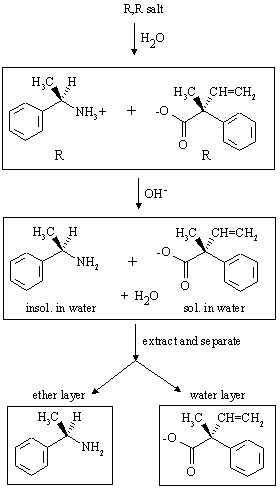
(c) What would you do to isolate the compounds present in the aqueous layers?
Answer: The compounds present in the aqueous layers can be isolated simply by acidifying the solution with aqueous HCl. This will protonate the carboxylate salts to generate the neutral acids, which are insoluble in water. If the neutral acid crystallizes, it can simply be collected by filtration. If it forms an oil or liquid, it would have to be recovered by extraction with ether.
(d) Explain what would happen if racemic 1-amino-1-phenylethane was used in the above procedure. Trace through each of the steps above, and draw perspective formulas of all compounds present at each stage.
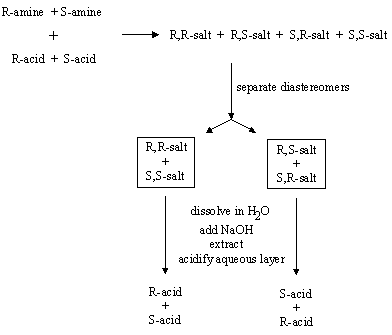
Answer: The experiment would appear to proceed in the same way. The final result though, would be two batches of racemic carboxylic acid rather than the "optically pure" enantiomers.
Four salts would be formed from reaction of the R- and S-amines with the R- and S-acids. As before, the R-amine would give the R,R and R,S salts. The S-amine would give the S,R and S,S salts. Your molecular models will verify that the R,R and S,S salts are enantiomers of one another, and that the R,S and S,R salts are enantiomers of one another.
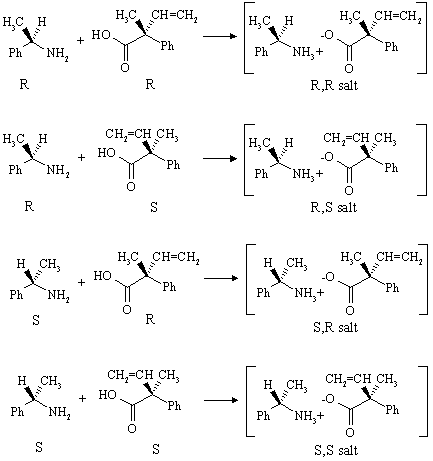
Thus, reaction of racemic amine with racemic acid gives two racemic diastereomers. These can be separated as before, but since they are racemic, racemic acids will be recovered after treatment with base and extraction:
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
26oct97; wjl