
| Problem Set #8 | ANSWERS | November 21, 1997 |
1. Synthesize the following from the compounds given. You may use any other reagent you like.

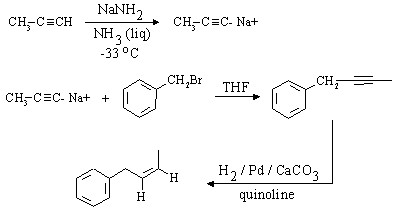
The desired compound is an E-alkene which contains 2 more carbon atoms than the alkyne given as starting material (phenylethyne). The synthesis must therefore contain a substitution reaction which uses a carbon nucleophile. E-alkenes can be synthesized from alkynes by dissolving metal reduction (Li or Na in liquid NH3); thus, a direct precursor to the final product is
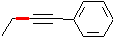
Comparing this structure to phenylethyne, we identify the C-C bond which must be formed (this is indicated in red in the structure above. The compound can thus be made by an SN2 substitution, using the phenylethynyl anion as nucleophile and bromo- or iodoethane as the electrophile. Be sure to use a dipolar aprotic solvent for this, as water and alcohols (or even acetone!) will protonate the strongly basic phenylethynyl anion. A complete synthesis is:
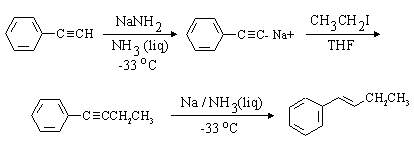
The desired compound is a Z-alkene which contains 3 more carbon atoms than the compound given as
starting material (bromomethylbenzene, or benzyl bromide). Again, a step involving nucleophilic substitution
with a carbon nucleophile will be required, as we need to make a carbon-carbon bond.
Z-alkynes can be synthesized from alkynes by catalytic reduction over poisoned catalysts, so a
direct precursor to the final compound is (as before, the C-C bond that needs to be formed is shown in red):
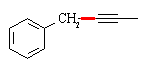
A complete synthesis would be:
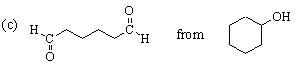
This one is easier. The desired compound, a 6-carbon dialdehyde, is the product of ozonolysis of cyclohexene using a reductive workup. Cyclohexene can be made from the given starting material (cyclohexanol) by dehydration. The complete synthesis is:
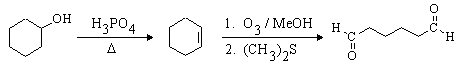
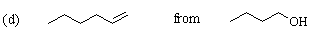
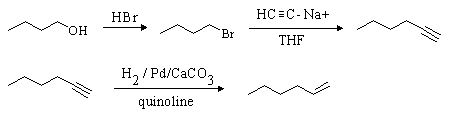
Careful! The desired product contains two more carbon atoms than the starting material. 1-Butanol must be converted to the tosylate or 1-bromobutane so that a 2-carbon unit can be added. As before, this can be done using sodium acetylide (HCC-Na+). The resulting compound (1-hexyne) can be reduced to 1-hexene either by hydrogenation with a poisoned catalyst or with Na / NH3:
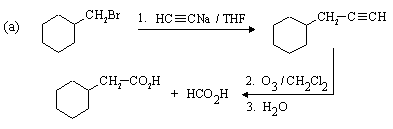
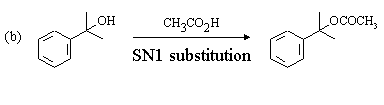
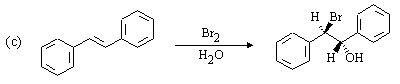
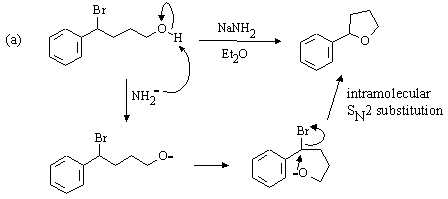
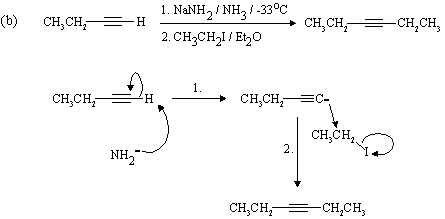
The Hahn-Ingold-Prelog nomenclature in the following question is challenging:
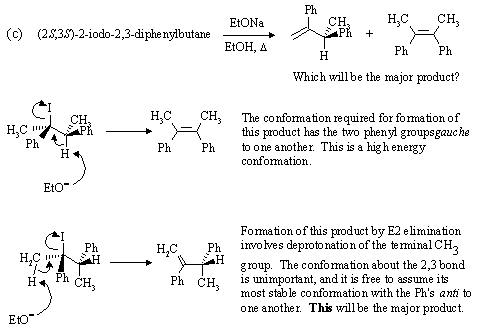
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
17nov97; wjl