Chem2O6
- 1997/98| Problem Set #9 | ANSWERS | December 7, 1997 |
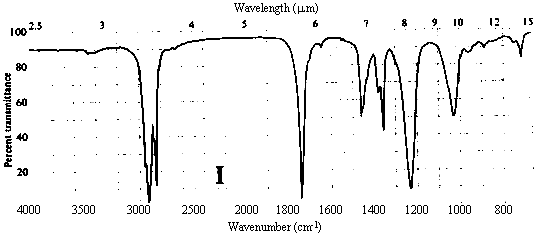
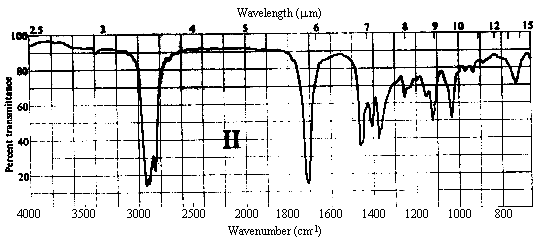
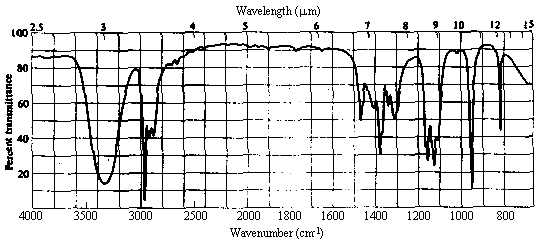
1. One of the compounds whose infrared spectrum is shown below is a ketone, and the other is an ester. Identify which spectrum belongs to which compound. Explain your reasoning.


Answer. The C=O stretching frequency in the infrared spectrum of an ester is typically about 30 cm-1 higher than that in the spectrum of a ketone of similar structure (see Table 10.2 in Ege). The really prominent difference, however, is the presence of a strong absorption band at 1000-1300 cm-1 in the spectrum of an ester due to the C-O single bond stretch. Spectrum I above contains strong bands at ~1720 and ~1200 cm-1, while II contains a strong band only at ~1690 cm-1. Thus, spectrum I belongs to the ester, while II belongs to the ketone.
2. Match each of the three 1H nmr spectra shown below with a compound in the following list (in the spectra, the number above each multiplet is the integral for that multiplet).
| (a) CH3CO2CH2CH3 (d) CH3CH2CH2NO2 |
(b) CH3CH2OCH3 (e) CH3CH2CH2CO2CH2CH3 |
(c) 2-chloropropene (f) iodoethane |
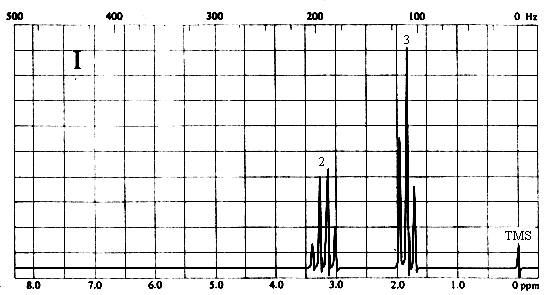
Two types of H's. The two-proton quartet at ~3.2 ppm suggests a CH2 group attached to a CH3 on one side and a relatively electronegative substituent on the other. The 3-proton triplet at ~2 ppm is due to the CH3 group. The only compound in the list which is consistent with this spectrum is iodoethane (CH3CH2I).
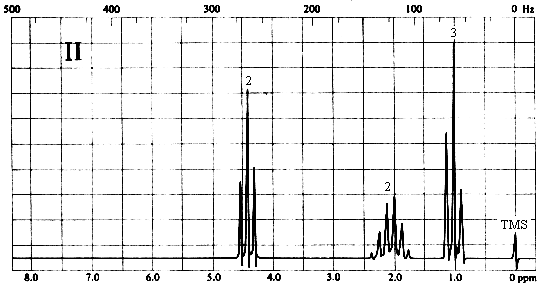
Three types of H's. The 2-proton triplet at ~4.4 ppm suggests a CH2 group attached to a CH2 on one side and a relatively electronegative substituent on the other. The 3-proton triplet at ~1 ppm is due to a CH3 group attached to a CH2. The 2-proton sextet at ~2.1 ppm suggests a CH2 group with 5 protons on its neighbouring carbons, or the central CH2 of an n-propyl (CH2CH2CH3) group. The only compound in the list which is consistent with this spectrum is (d), 1-nitropropane.
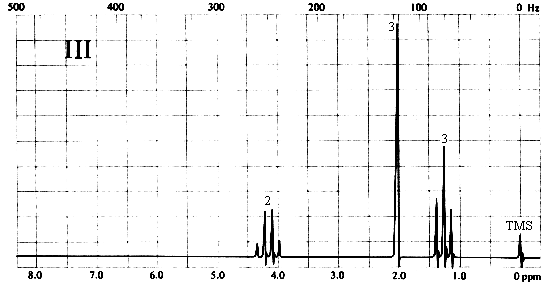
Three types of H's. The 3-proton singlet at ~2 ppm is due to a CH3 group with no neighbouring protons. The chemical shift is characteristic of a CH3C=O group. The 2-proton quartet at ~4.2 ppm suggests a CH2 group attached to a CH3 on one side and a relatively electronegative substituent (such as oxygen) on the other. The 3-proton triplet at ~1.3 ppm is due to the CH3 group. The only compound in the list which is consistent with this spectrum is (a), ethyl acetate. Note that ethyl methyl ether (b) would also give rise to a spectrum containing a 3-proton singlet, a 2-proton quartet, and a 3-proton triplet; however, the singlet would be expected to appear at ~3.5 ppm, right beside the quartet at ~4 ppm.
3. The infrared and 1H nmr spectra of a compound with molecular formula C3H8O are shown below. What is the structure of this compound?
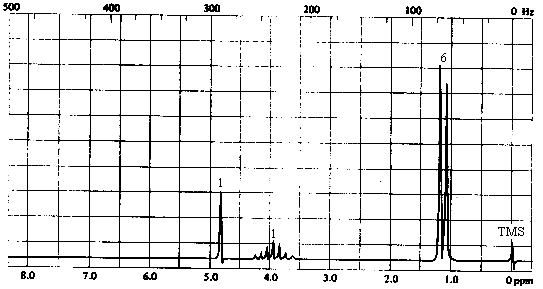
| 1. Molecular Formula | C3H8O -> 0 sites of unsaturation. Must be an aliphatic alcohol or ether. |
| 2. Infrared Spectrum | - strong broad peak at ~3400 cm-1 -> OH of an alcohol. |
| 3. 1H NMR spectrum | - 3 types of H's: - singlet at 4.2 ppm (1H) -> most likely the OH proton. Except in very dry, very pure solvents, OH protons do not show coupling to neighbouring protons because of rapid exchange. - doublet at ~1.2 ppm (6H) -> six equivalent protons with 1 nearest neighbour. A 3-proton doublet in the 1-2 ppm range indicates a CH3CH- group; a 6-proton doublet in the same range indicates a (CH3)2CH- group. - septet at ~3.9 ppm (1H) -> a single proton with 6 nearest neighbours: the CH proton of the -CH(CH3)2 group. |
The compound can only be 2-propanol, (CH3)2CHOH.
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
07dec97; wjl