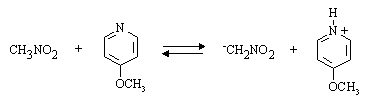
| Class Test #1 | ANSWERS | October 30, 1996 |
1. When nitromethane (pKa 10.2) and 4-methoxypyridine (pKa 7.0) are mixed together in water, the following acid-base equilibrium is established:
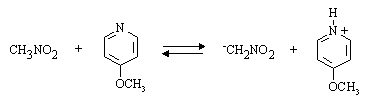
(a) Draw complete Lewis line-dot structures for ...
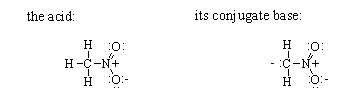
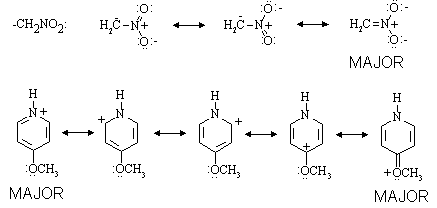
(b) Draw resonance structures for the two ions on the right-hand side of the equilibrium, and for each, indicate which are major contributors.
(c) For the -CH2NO2 anion, identify the hybridization of the ... carbon: sp2 ... nitrogen: sp2 ... oxygens: sp2 and sp2.
(d) What is the orientation of the lone pair of electrons on the nitrogen atom of 4-methoxypyridine with respect to the plane of the ring? in the plane (parallel)
(e) Does the equilibrium lie on the right-hand side (RHS) or the left-hand side (LHS)? LHS
(f) The standard free energy for this reaction is ![]() Go = +18.2 kJ/mol at 25 oC.
Go = +18.2 kJ/mol at 25 oC.
Calculation:
![]() Go = -RTln Keq = -2.303RTlog Keq
Go = -RTln Keq = -2.303RTlog Keq
Keq = KaNM / KaMP (NM = nitromethane; MP = 4-methoxypyridine)
log Keq = log KaNM - log KaMP
= pKaMP - pKaNM
= -3.2
![]() Go = -2.303(0.0083)(298)(-3.2) = 18.2 kJ/mol.
Go = -2.303(0.0083)(298)(-3.2) = 18.2 kJ/mol.
(i) complete the Lewis structures for the pertinent parts of the molecules,
(ii) label the nucleophiles ("N") and electrophiles ("E"), and
(iii) draw curly arrows showing the movement of electrons that take place to convert reactants to products.
See: Ege, Problem 4.21(b)
(a) 1-chloro-3-ethyl-4-methylpentane
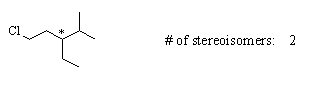
(b) 4-(1-methylethyl-5-methyl-2-phenyl-3-hexanol
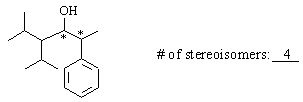
(a) Draw the two chair conformations of fluorocyclohexane and indicate by the length of arrows between the two, which conformer predominates at equilibrium.
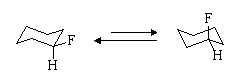
(b) Draw the two chair conformations of trans-1-fluoro-3-methylcyclohexane and indicate by the length of arrows between the two, which conformer you would expect to predominate at equilibrium.
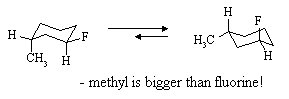
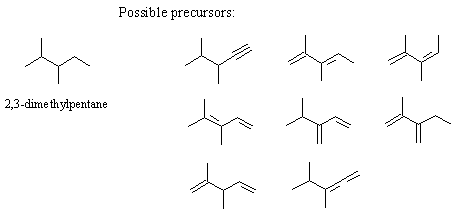
6. (a) A compound with the molecular formula C7H12 has 2 sites of unsaturation. Thus, the molecule contains either 1 triple bond , 2 double bonds , 1 ring and 1 double bond , or 2 rings (there are four possibilities). For each of the four categories of compounds given as possibilities for the molecular formula C7H12, draw one structural formula.

(b) Adding 2 moles of hydrogen to the compound gives 2,3-dimethylpentane. In light of this evidence, what structures are possible for the C7H12 compound?
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
12nov96; wjl