| Assignment #2 | Due: November 11, 1996 |
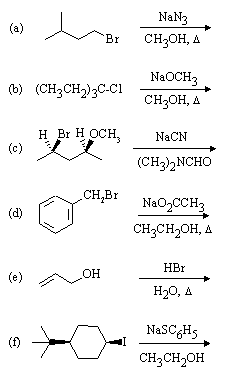
1. Predict the principal products of the following reactions, showing stereochemistry where appropriate.

2. Suggest a synthesis of (R)-2-butanol from (R)-2-bromobutane. Two steps may be required.
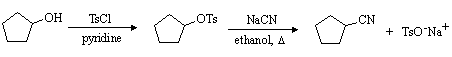
(a) Write a mechanism for the nucleophilic substitution reaction in the equation above.
(b) Carboxylic acid esters are synthesized by a similar procedure to that used for the synthesis of sulfonate esters. For example,
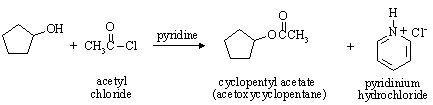
Write a reasonable mechanism for the formation of cyclopentyl acetate from cyclopentanol and acetyl chloride, based on your knowledge of sulfonate ester formation.
(c) What would be the product of reaction of (1R,2S)-2-methylcyclopentanol with acetyl chloride in pyridine? (Give the structure and name)
(d) While tosylate and mesylate are very good leaving groups in nucleophilic substitution reactions, acetate and other carboxylate anions are not. Explain why.
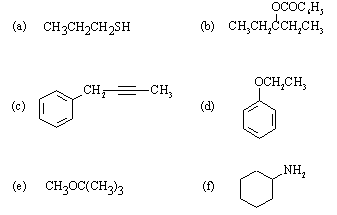
Make sure that your answers are STAPLED TOGETHER and labelled with:
Your NAME & STUDENT NO.
Assignments are due at 5pm on Monday, November 11
(in your Tutorial Leader's DropBox in ABB).
Late assignments will receive a grade of ZERO, unless a medical slip is filed with the Dean's Office.
01nov96; wjl
Your LAB-SECTION/GROUP/TA (Repeaters should indicate their Tutorial Section)
Go to:
Instructions for Printing this Document
Chem2O6 Problem Sets & Answers
Chem2O6 Home Page.