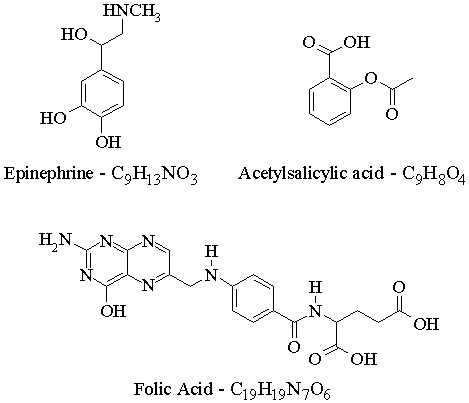
| Answers to Assignment #1 |
1. Convert the following line structures into molecular formulas.

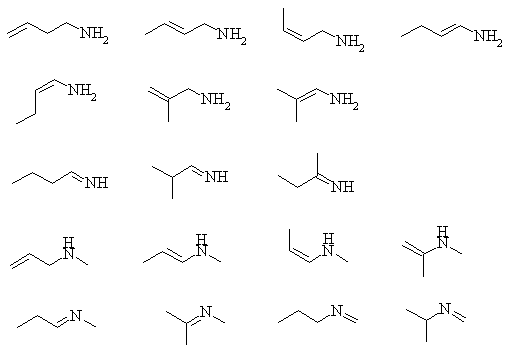
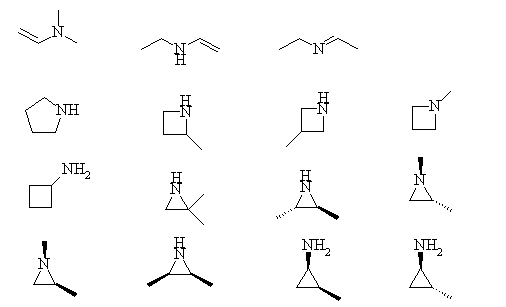
The bases, in order of decreasing basicity are:
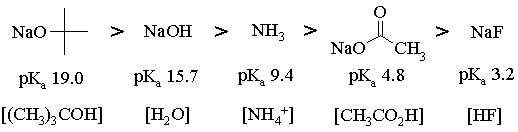
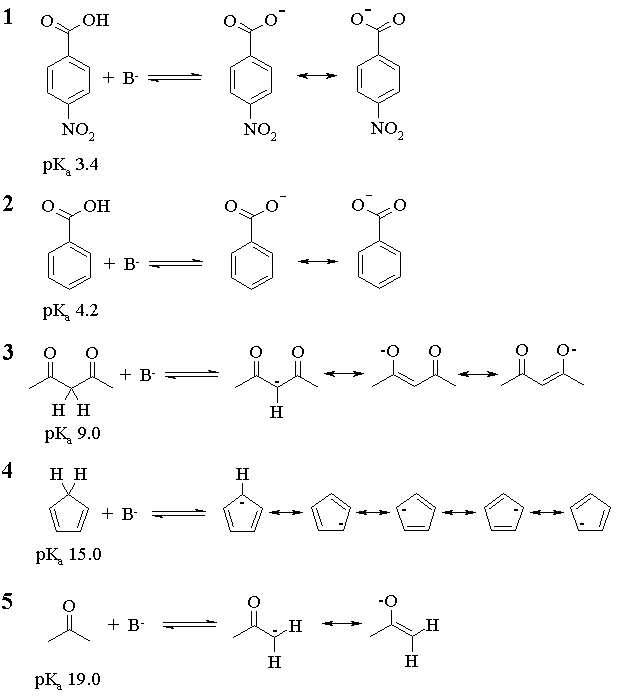
For an equimolar mixture of any acid-base pair, the acid will be more than 50% deprotonated when the pKa of the acid is less than or equal to the pKa of the conjugate acid of the base. Thus,
For 1, all bases but NaF will work (NaF will almost get us there, though);
For 2, all but NaF will work;
For 3, NaOC(CH3)3, NaOH and NH3 will work;
For 4, NaOC(CH3)3 and NaOH will work;
For 5, only NaOC(CH3)3 will work.
(b) Acid-base reactions are generally carried out in a polar solvent so as to dissolve the ions involved in the equilibrium.
All of the above reactions except the last one could be carried out in water or methanol. This is because NaOC(CH3)3 is a stronger base than hydroxide; thus, when it is dissolved in water, it is almost quantitatively protonated by the solvent to produce HOC(CH3)3 and NaOH. NaOH has pKa 15.7 and is not strong enough to deprotonate acid 5.
This is called the solvent levelling effect: in any solvent, the strongest base that can exist is the conjugate base of the solvent.
Keq = Ka (acid) / Ka (base)
Therefore, log Keq = pKa (base) - pKa (acid)
In this case, log Keq = 9.4 - 7.2 = 2.2
Therefore, Keq = 158
(b) Keq = exp(-![]() Go/RT)
Go/RT)
= exp(-7.3/{(.00831)(298)})
= exp(-2.95)
= 0.0525
(c) at 298K, Keq = [A]/[E] = 0.0525
[A] + [E] = 100%
.0525[E] + [E] = 100%
[E] = (100 / 1.0525)% = 95%
(d) Because the equilibrium involves only a conformational change, ![]() So ~ 0. Therefore
So ~ 0. Therefore ![]() Go is temperature independent.
Go is temperature independent.
For a mixture consisting of 48% A, Keq = [A]/[E] = 48/52 = 0.923.
Since ![]() Go = -RTlnKeq,
Go = -RTlnKeq,
T = -![]() Go / (RlnKeq)
Go / (RlnKeq)
= -7.3/(.00831*ln(0.923))
= 10,974 K !
H2C=CHCH3 + Br2 --> H2CBr-CHBrCH3
Bonds made = 2(C-Br) + 1(C-C)
Bonds broken = 1(C=C) + 1(Br-Br)
![]() Hr = 2DE(C-Br) + DE(C-C) - {DE(C=C) + DE(Br-Br)}
Hr = 2DE(C-Br) + DE(C-C) - {DE(C=C) + DE(Br-Br)}
![]() Hr ~ 2(68) + 83 - {146 + 46}
Hr ~ 2(68) + 83 - {146 + 46}
= -27 kcal/mol
For the free radical bromination of propene,
H2C=CHCH3 + Br2 --> H2C=CHCH2Br + HBr
Bonds made = 1(C-Br) + 1(H-Br)
Bonds broken = 1(C-H) + 1(Br-Br)
![]() Hr = DE(C-Br) + DE(H-Br) - {DE(C-H) + DE(Br-Br)}
Hr = DE(C-Br) + DE(H-Br) - {DE(C-H) + DE(Br-Br)}
![]() Hr ~ 68 + 87 - {99 + 46}
Hr ~ 68 + 87 - {99 + 46}
= -10 kcal/mol
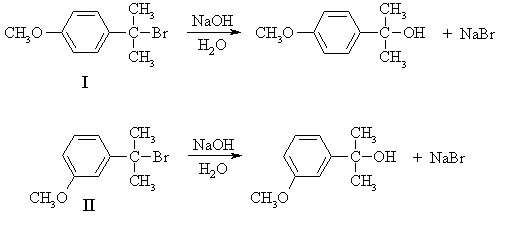
Since the rate does not depend on the concentration of the nucleophile, the mechanism is SN1:
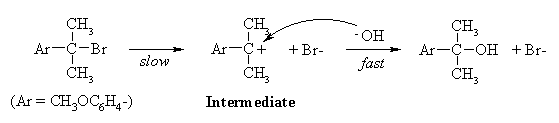
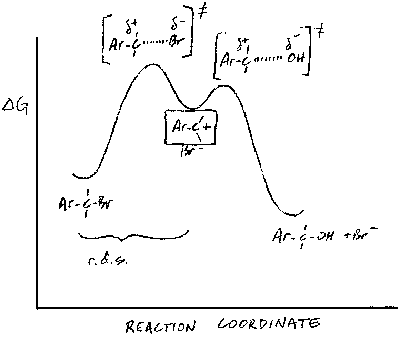
(b) Since the rate of the reaction depends only on that of the first step - formation of the carbenium ion intermediate - the answer must lie in the relative stabilities of the intermediates formed from the two compounds. In strongly endothermic processes such as the formation of carbenium ions, the transition state contains a lot of the character of the product, because the transition state is closer to the product than the reactant on the reaction coordinate. Thus, as the product becomes more stable, the transition state energy is lowered and the rate increases.
The carbenium ions formed from I and II are both 3o and hence identical in structure at the positively-charged carbon. However, they are more stable than simple 3o alkyl carbenium ions because of resonance with the aromatic ring. Is there any difference in the resonance structures that can be drawn for the two ions?
The resonance structures of a phenyl-substituted carbenium ion indicate that positive charge is delocalized into the "ortho" (2 and 6) and "para" (4) positions of the aromatic ring. There is no positive charge on the "meta" (3 and 5) positions:
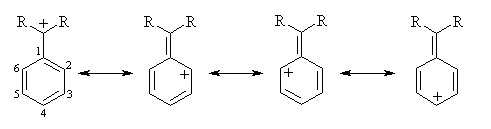
Thus, if a methoxy substituent is placed in the ortho or para positions, it can further delocalize positive charge using the lone pairs on oxygen; it can't when it's placed in a meta position (ignoring charge-separated resonance structures):
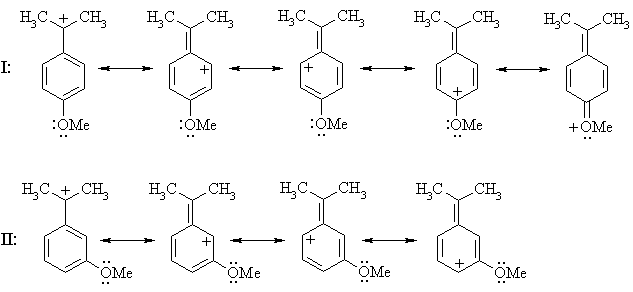
The carbenium ion from I enjoys additional stabilization by the methoxy substituent while that from II doesn't, so the ion from I is the more stable and hence will be formed faster; i.e. I reacts faster than II.
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
14oct97; wjl