| Assignment #1 | Due: October 10, 1997 |
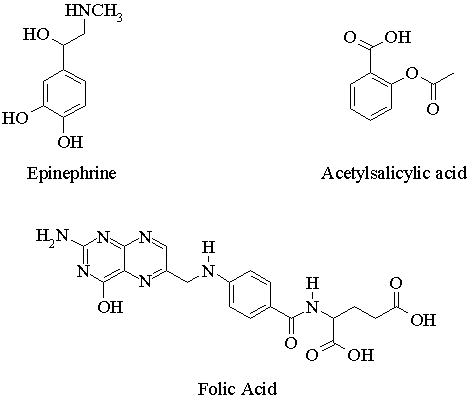
1. Convert the following line structures into molecular formulas.

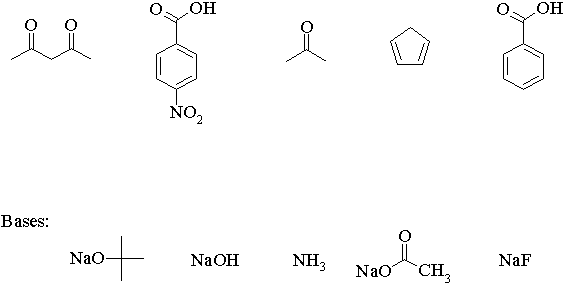
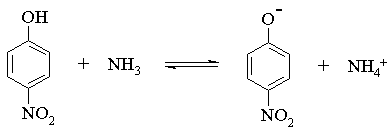
(b) In most cases, acid-base reactions must be carried out in a polar solvent like water, an alcohol, or an ether. Why? Could you carry out all of the above deprotonation reactions in methanol or water? If no, identify which ones wouldn't work, and explain why.
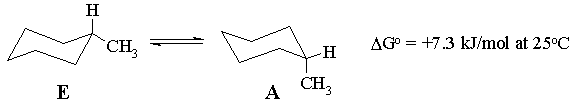
(a) Which conformer will predominate at 25 oC?
(b) Calculate the equilibrium constant at 25 oC, for the equilibrium as written above.
(c) Calculate the percentages of A and E present at 25 oC.
(d) Calculate the temperature at which the mixture would consist of 48% A and 52% E.
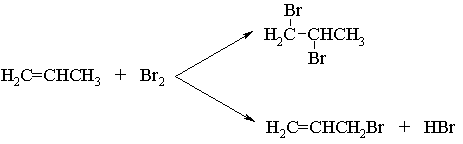
Using the table of average bond energies on p.67 of the textbook, estimate ![]() Hr for the two possible reactions, and identify which of the two is more exothermic.
Hr for the two possible reactions, and identify which of the two is more exothermic.
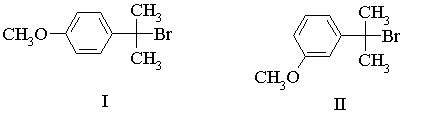
(a) Identify the products expected from these reactions. Using one of the compounds, draw the reaction coordinate diagram for the reaction, and draw structures for all energy minima and maxima. Identify the transition state for the rate determining step.
(b) One of the compounds reacts much faster than the other. Identify which one you would expect to be more reactive, and explain why.
Make sure that your answers are STAPLED TOGETHER and labelled with:
Your NAME & STUDENT NO.
Assignments are due at 5pm on Friday, October 10 (in your DropBox in ABB - the one corresponding to your tutorial section).
Late assignments will receive a grade of ZERO, unless a medical slip is filed with the Dean's Office.
02oct97; wjl
Your LAB-SECTION/GROUP/TA
Go to:
Instructions for Printing this Document
Chem2O6 Problem Sets & Answers
Chem2O6 Home Page.