Write in the assignment of the various resonances in the space provided in the table above.
Chem2O6
- 1997/98| Assignment #4 | Answers | Due: February 9, 1998 |
1. You are given a compound with the molecular formula C4H8O3
and are asked to identify its structure. You rush to the NMR lab and obtain the proton and
carbon spectra and then tabulate the data as shown below.
Proton NMR Carbon NMR
ppm |
|
|
|
ppm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

Write in the assignment of the various
resonances in the space provided in the table above.
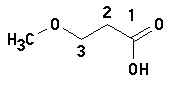
3-methoxy-propanoic acid
The key feature would be bands associated with the carboxylic acid group. These would include a large absorption 1700 to 1720 cm-1 due to the C=O bond stretch and also a broad featureless band associated with the OH group ranging from ca 3400 to 2700 cm-1.
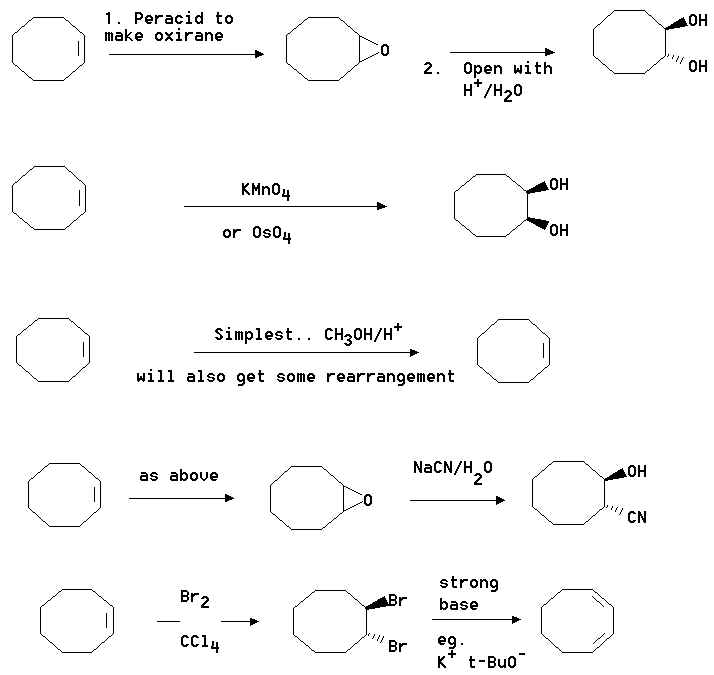
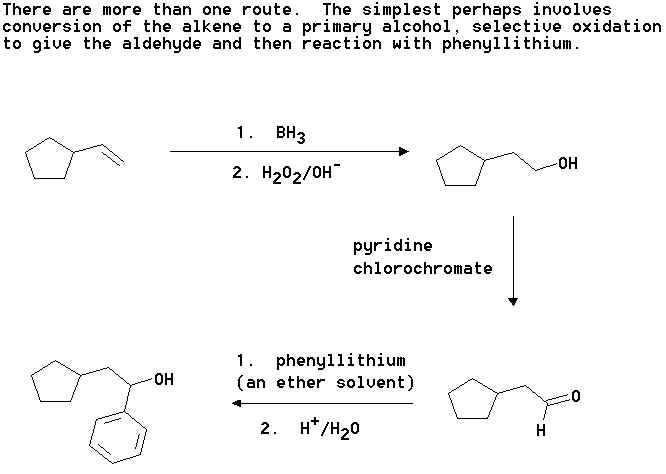
2. Show how you would convert cyclooctene into the following compounds. Where you
propose more than one step clearly show the structure of any intermediate compound.
3. Propose a mechanism for the following acid catalysed transformation. (The acid is a trace of concentrated sulphuric acid added to the methanol solvent.)
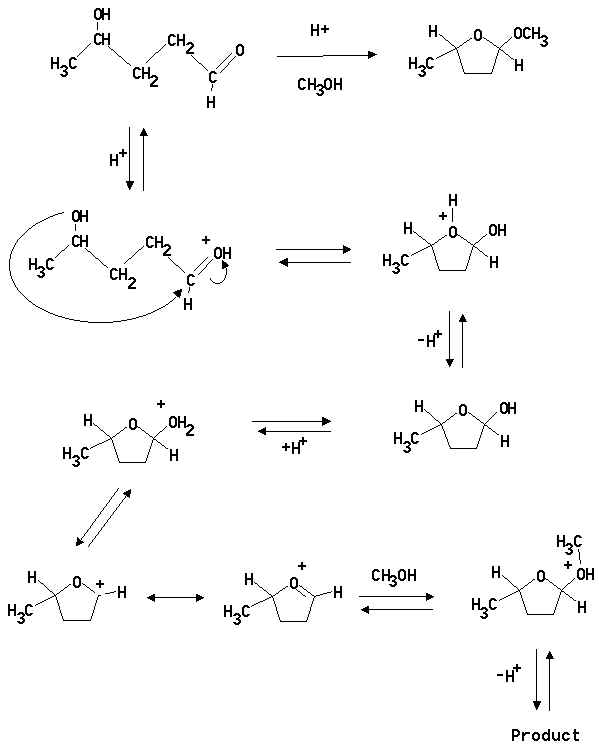
Note: It is possible to write a mechanism forming the hemiacetal or acetal first. Such an answer is correct.
Note: Answers in which an alkoxide ion is used to attack the carbonyl carbon are incorrect.
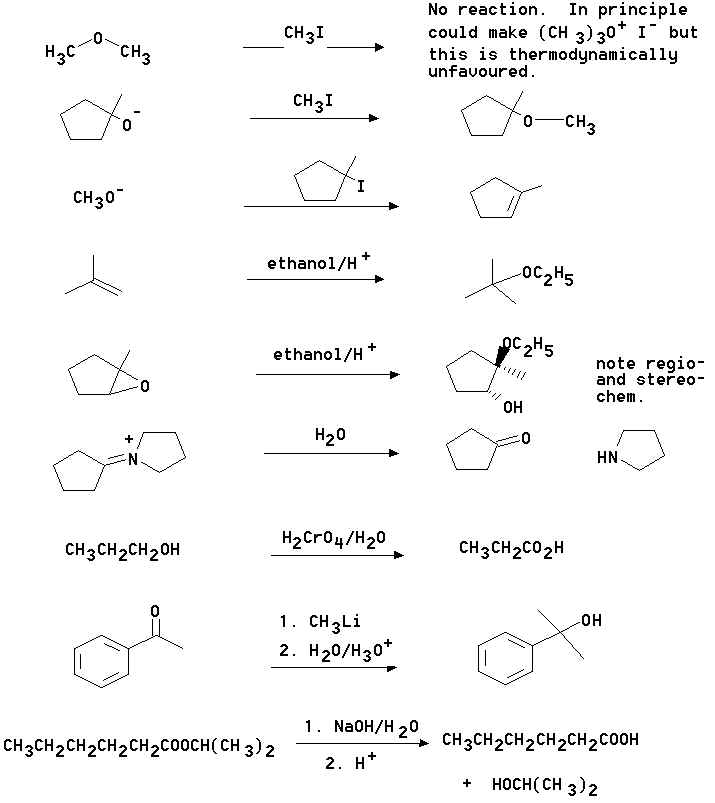
4. What are the principal products of the following reactions?
5. Show how you could accomplish the following synthetic transformation. Note, more than one step is involved. Clearly show the structures of any proposed intermediary compounds and the reagents/conditions used in each step you propose.
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
30jan98; jp