Chem2O6
- 1997/98| Assignment #4 | Due: February 9, 1998 |
1. You are given a compound with the molecular formula C4H8O3 and are asked to identify its structure. You rush to the NMR lab and obtain the proton and carbon spectra and then tabulate the data as shown below.
Proton NMR Carbon NMR
Proton Shift ppm |
Multiplicity |
Integration |
Assignment |
Carbon Shift ppm |
Assignment |
2.62 |
Triplet |
2 |
34.75 |
||
3.38 |
Singlet |
3 |
58.72 |
||
3.68 |
Triplet |
2 |
67.55 |
||
11.5 |
Singlet |
1 |
177.33 |
What is the structure of the compound?
Write in the assignment of the various resonances in the space provided in the table above.
Name the compound.
You are now off to confirm your assignment by running an infrared spectrum. What key features would expect in your ir spectrum?
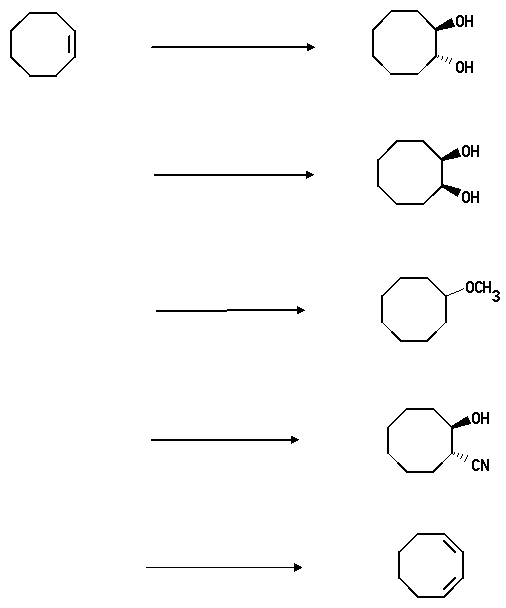
2. Show how you would convert cyclooctene into the following compounds. Where you propose more than one step clearly show the structure of any intermediate compound.

3. Propose a mechanism for the following acid catalysed transformation. (The acid is a trace of concentrated sulphuric acid added to the methanol solvent.)
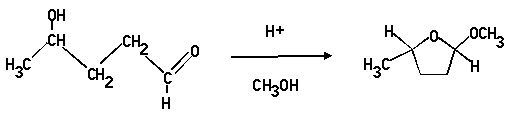
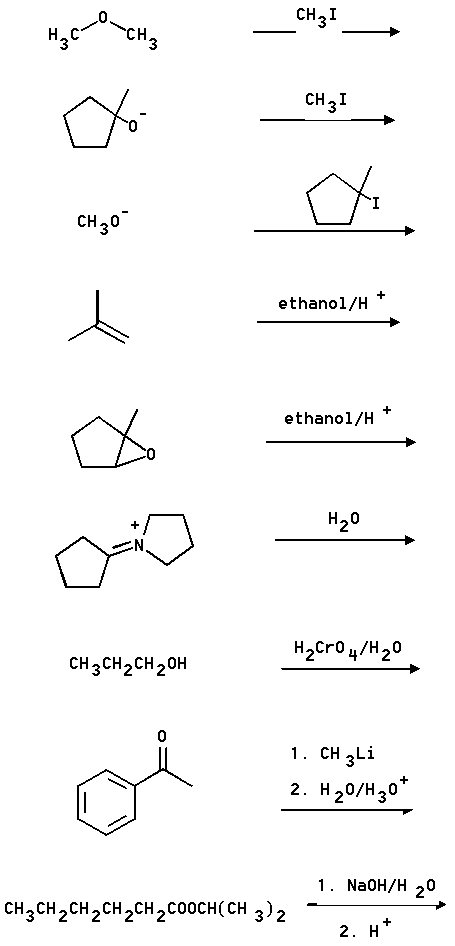
4. What are the principal products of the following reactions?
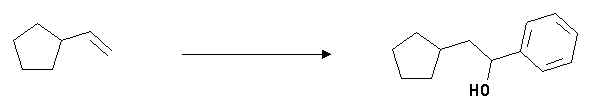
5. Show how you could accomplish the following synthetic transformation. Note, more than one step is involved. Clearly show the structures of any proposed intermediary compounds and the reagents/conditions used in each step you propose.
Make sure that your answers are STAPLED TOGETHER and labelled with:
Your NAME & STUDENT NO.
Your TUTORIAL LEADER
Your LAB-SECTION/GROUP/TA (Repeaters should indicate their Tutorial Section)
Assignments are due at 5pm on Monday, February 9 (in your Tutorial Leader's DropBox in ABB). Late assignments will receive a grade of ZERO, unless a medical slip is filed with the Dean's Office.
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
30jan98; jp