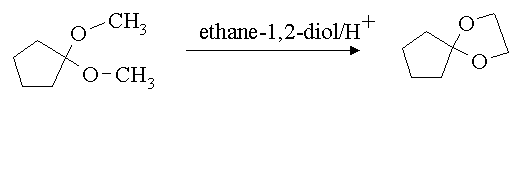
| Problem Set #11 | Answers | January 23, 1998 |
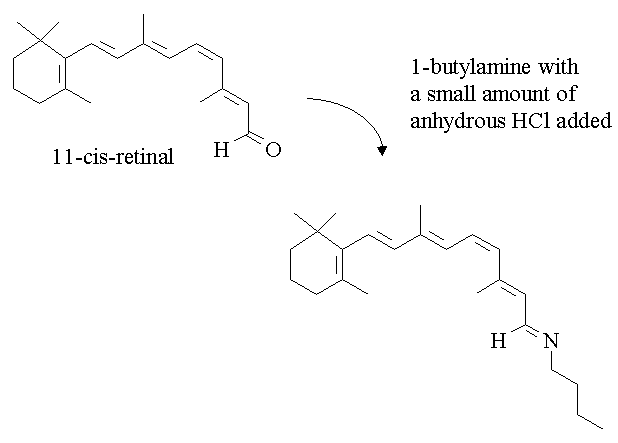
1. Write a mechanism for the following reaction.
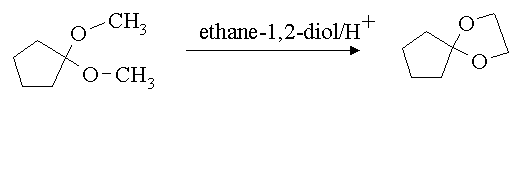
Answer:>
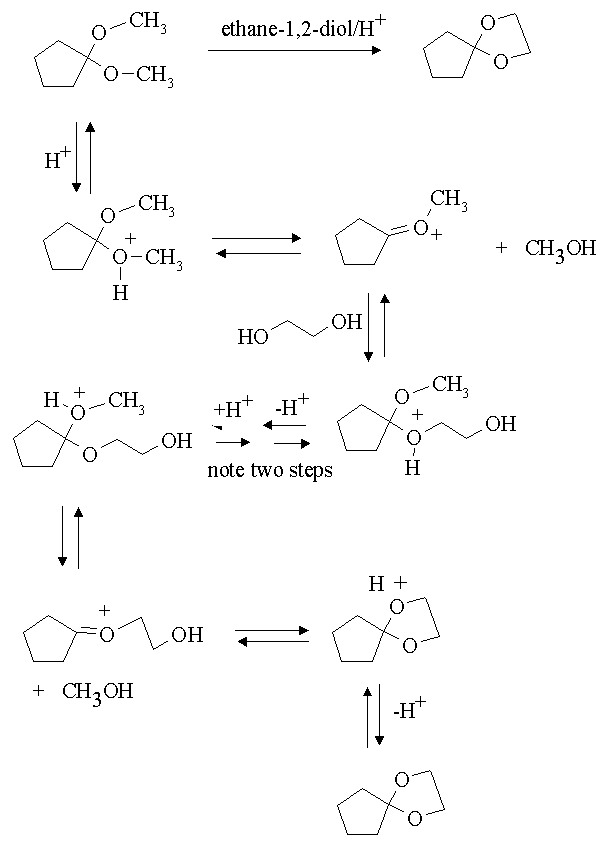
2. How would you use a Grignard reaction with an aldehyde or ketone to make the following compounds?
a) 2-pentanol
b) 1-butanol
c) diphenylmethanol
Answer:
I have shortened the following answers to not include the making of the Grignard reagent in a couple of cases. Your answers should include this. The acid work-up is as you did in the lab.
Note that there can be alternative answers in most cases.
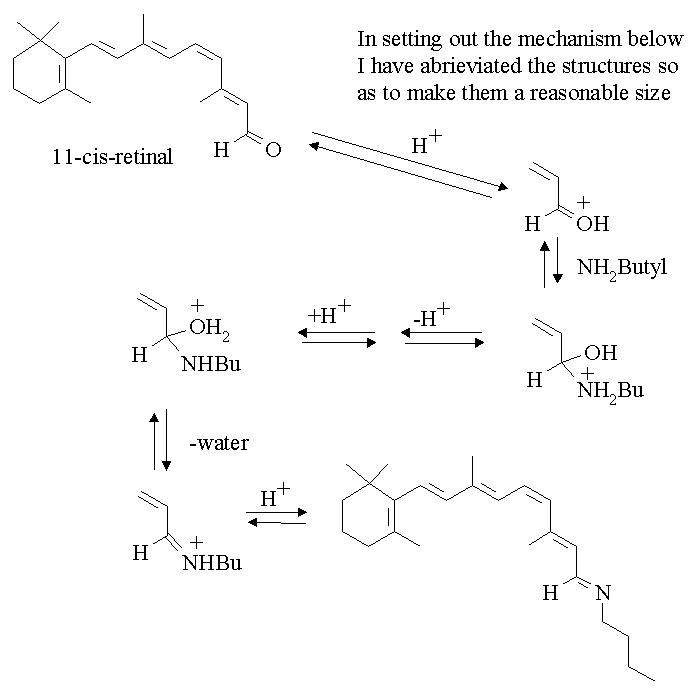
3. Write a mechanism for the following reaction.
Answer:
In this answer I have not involved the equilibrium between butylamine and HCl. This will of course be occurring as a fast reaction. Thus loss of a proton will be to a free butylamine. The proton source will be the protonated butylamine.
4. Show how you would carry out the following transformations.
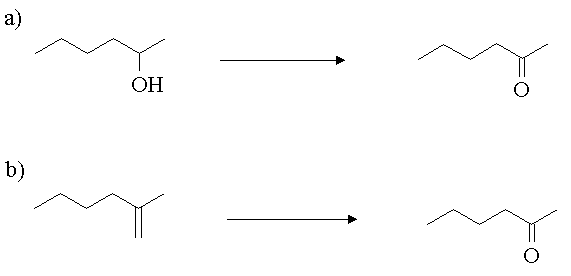
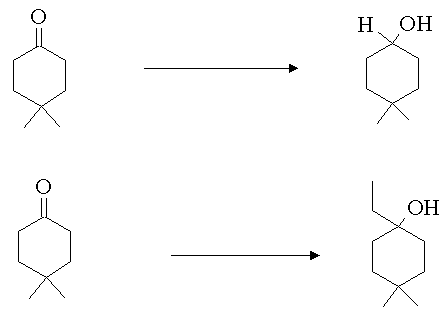
Answer:
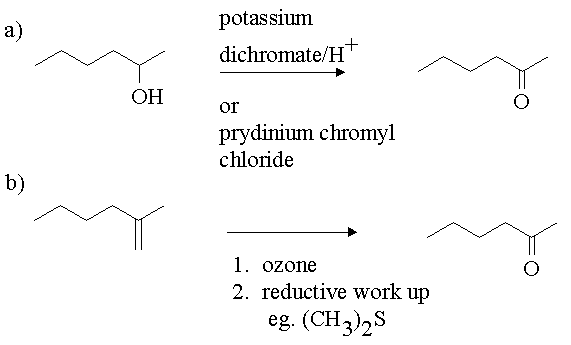
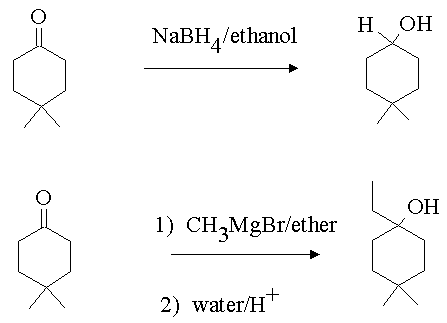
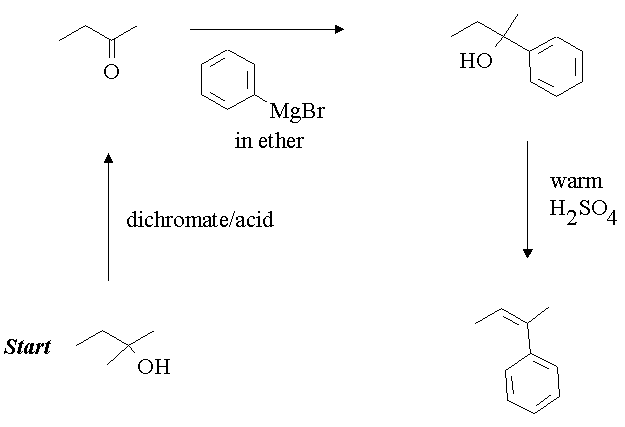
5. You have been given a sample of 2-butanol. You have need of 2-phenyl-2-butene for a specific application. How would you convert the 2-butanol to the desired material. You may use any reagents you wish including other carbon containing compounds.
Answer:
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
23jan98; jp