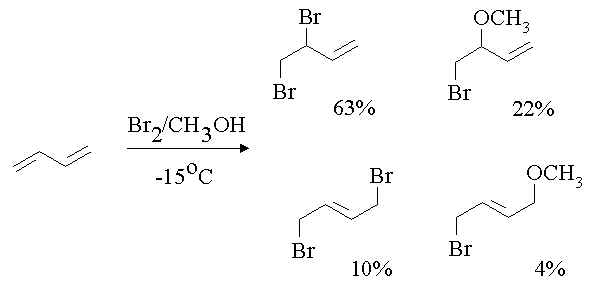
1. Propose a mechanism to account for the following products.
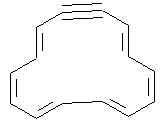
2. Professor T. W. O. O’Six prepared the following molecule. Before making it he counted the number of -electrons and figured it would not be stable. In fact it was stable, surprise, surprise. Where did the professor make his mistake? Show the electrons involved in cyclic delocalization in the compound.
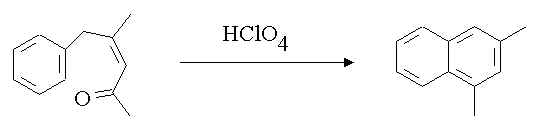
3. Write a mechanism for the following reaction.
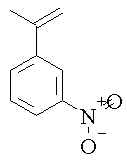
4. What is the structure of imidazole? Draw a sketch of imidazole showing the the atomic orbitals used to make up the -system and nitrogen lone pairs. Use this to predict which nitrogen in imidazole would be protonated when the molecule is treated with acid.
5. Starting from benzene show how you would prepare -
6. Butanal reacts in the presence of base in water to form 2-ethylhex-2-enal.
A. Write out the structures of butanal and 2-ethylhex-2-enal.
B. Suggest a mechanism for the conversion.
C. 2-Ethylhexanol can be produced from 2-ethylhex-2-enal. How would you carry out this
transformation.?
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
20 mar 98; jp