
Chem2O6
- 1997/98| Problem Set #2 | ANSWERS | September 26, 1997 |
1. Write the following condensed formulas as Lewis structures:
(i) (CH3)3SO+ (ii) CH2N2 (iii) CH3NHCONH2 (iv) CH3CH2N3 (v) CHCCHNCH3
Answer:

2. Write resonance structures for the following, indicating major and minor contributors (C6H5 is a phenyl ring).
(i) [CH3CHOCHCH2]+ (ii) [CH3CH2OCHCHCH2]+ (iii) [C6H5CH2]- (iv) [CH3COCH2]-
(v) [CH2CHCHCHCH2]+ (vi) [C6H5CHNH2]+
(vii) [CH2CHCH2]
Answer:


3. Draw a molecular orbital diagram for carbon tetrachloride (CCl4), using sp3 hybrid atomic orbitals for carbon and a 3p atomic orbital for each of the chlorines. Fill the MO's with the eight electrons involved in sigma-bonding in the molecule. Draw pictorial representations of one of the bonding and one of the antibonding MO's.
(a) Irradiation of a solution of CCl4 in cyclohexane (C6H12) with UV light results in the formation of the following products:
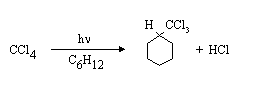
Suggest a mechanism for the reaction, and explain what the role of the UV light is, using your MO diagram for assistance.
(b) Reduction of carbon tetrachloride with sodium metal leads to the formation of hexachloroethane (C2Cl6) and sodium chloride. Suggest a mechanism for this reaction, again using MO diagrams to help explain why the reaction occurs.
Answer:
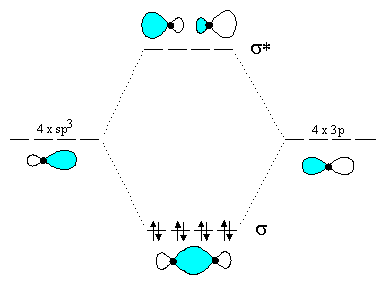
Note that only one of the C-Cl bonding MO's and one of the C-Cl antibonding MO's are shown.
(a) UV excitation of CCl4 causes promotion of an electron from a ![]() -MO to a
-MO to a ![]() * (antibonding) MO. This
causes rupture of a C-Cl bond, forming chlorine atoms and trichloromethyl radical. The
chlorine atom abstracts hydrogen from the solvent (cyclohexane), forming HCl and
cyclohexyl radical. The cyclohexyl and trichloromethyl radicals combine to form
trichloromethylcyclohexane. The actual product distribution is far more complex than this
due to other free radical reactions.
* (antibonding) MO. This
causes rupture of a C-Cl bond, forming chlorine atoms and trichloromethyl radical. The
chlorine atom abstracts hydrogen from the solvent (cyclohexane), forming HCl and
cyclohexyl radical. The cyclohexyl and trichloromethyl radicals combine to form
trichloromethylcyclohexane. The actual product distribution is far more complex than this
due to other free radical reactions.

(b) While we discussed this kind of thing in lectures, this question is a challenging
one. Reduction of CCl4 by sodium yields Na+ and the anion radical
of carbon tetrachloride. The extra electron must go into a ![]() *
MO of CCl4. This causes a C-Cl bond to cleave, yielding chloride ion and
trichloromethyl radical. Chloride and sodium ions combine to yield NaCl, while two
radicals combine to give hexachloroethane. Note that the balanced equation requires
2 Na and 2 CCl4 on the reactant side, and 2 NaCl and 1
Cl3C-CCl3 on the product side.
*
MO of CCl4. This causes a C-Cl bond to cleave, yielding chloride ion and
trichloromethyl radical. Chloride and sodium ions combine to yield NaCl, while two
radicals combine to give hexachloroethane. Note that the balanced equation requires
2 Na and 2 CCl4 on the reactant side, and 2 NaCl and 1
Cl3C-CCl3 on the product side.

4. Draw a molecular orbital diagram for water (H2O), using two hydrogen 1s atomic orbitals and four oxygen sp3 hybrid orbitals. Fill the MO's with the eight electrons.
Why would it be incorrect to construct the MO's using sp hybrid atomic orbitals for the O-H bonds, and two pure p-orbitals for the lone pairs?
Answer:
Six atomic orbitals (4 on the oxygen, 1 each from the two hydrogens) must combine to
form six molecular orbitals. Since there are only two bonds in the molecule, there
can only be two bonding MO's (and two antibonding MO's). Two of the sp3
hybrid orbitals on oxygen are not involved in bonding - they are nonbonding, and
their energies will stay the same. The MO's are then filled with the 8 electrons; 4 go in
the ![]() -MO's and 4 go in the n-MO's.
-MO's and 4 go in the n-MO's.

The atomic orbitals that one uses for the construction of molecular orbitals are chosen based on the known geometry of the molecule. Thus, the choice of sp3 hybrid AO's for oxygen was made because the H-O-H bond angle is 104.5o, close to the tetrahedral angle. One would choose to use sp hybrids only when the molecule is linear.
| Go to: | Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
25sep97; wjl