| Solving Spectral Problems | November 21, 1997 |
The following is an example of the typical sort of approach that one uses to solve spectral problems. What you should learn from it is a sense of the logic that's involved, not the order in which you consider the data (especially that in the nmr spectrum). It is most important that you work on developing your own general approach to solving a problem - as usual, this can only be accomplished through practice. There are lots of opportunities for this between the problems in Chapter 11 of Ege, Problem Set 9, and Assignment 3.
First, qualitatively examine all the data given, and extract any information that "jumps out" at you. Then proceed to a more detailed inspection of the data.

| 1. Molecular Formula | C10H12O2 -> 5 sites of unsaturation. For a molecule this size,
(i.e., only ten carbons), such a large number of sites of unsaturation is usually due to the presence
of a phenyl ring (which provides 4 sites on its own). For a quick proof, take a quick glance at the
1H nmr spectrum, and look for signals in the 7-8 ppm (aromatic proton) region. No question, they're there!
This leaves only one site unaccounted for. |
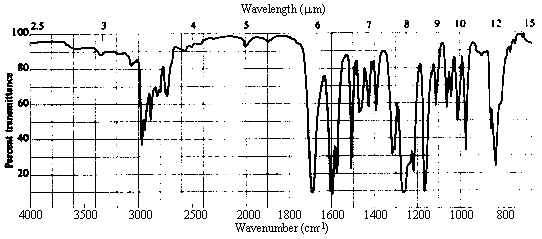
| 2. Infrared Spectrum | - strong peak at ~1700 cm-1 -> C=O (this accounts for the other site of unsaturation!) - nothing at ~3500 cm-1 -> no OH, so a carboxylic acid or alcohol can be ruled out.
- weak absorption in the 3000-3100 cm-1 region indicative of sp2 C-H stretches, in addition to
prominent bands in the 2800-3000 cm-1 range due to sp3 C-H. There is lots more information here,
but this is the easiest to pull out, and all we need for the present. |
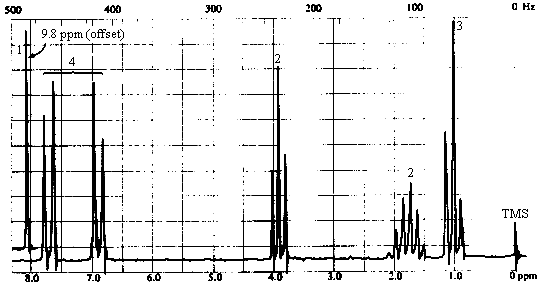
| 1H NMR spectrum | - 6 types of H's: - singlet at 9.8 ppm (1H) -> aldehyde or carboxylic acid proton. The ir spectrum rules out a carboxylic acid, so this must be an aldehyde. The absence of coupling indicates that the -CHO group is bonded to an atom with no protons attached to it. - two doublets at ~6.9 and ~7.7 ppm (4H) -> aromatic protons; symmetrical pattern of two doublets suggests a 1,4-disubstituted benzene. Each doublet corresponds to 2 equivalent protons, each of which has one nearest neighbour:
Note that the two doublets are skewed towards one another and that the magnitude of the splitting is the same for both - this verifies that the two doublets "belong together"; i.e., they represent a pair of protons coupled to each other. We've accomplished alot; check the molecular formula to see what's left! We've accounted for a -CHO group and a -C6H4-. All that's left is three carbons bearing 7 hydrogens and an oxygen. Since all sites of unsaturation are accounted for, the seven H's must all be bound to sp3 carbons. - triplet at ~4 ppm (2H) -> two equivalent protons with 2 nearest neighbours. With only 3 carbons left, this must be due to a CH2 attached to a CH2! - triplet at 1 ppm (3H) -> three equivalent protons with 2 nearest neighbours. This must be due to a methyl group attached to a CH2. The last two bits of information, coupled with the fact that we have only C3H7 left, are only consistent with one structure - CH2CH2CH3!
- sextet at ~1.8 ppm (2H) -> two equivalent protons with 5 nearest neighbours. This is the
middle CH2 in the -CH2CH2CH3 chain. |
Let's take stock of what we have:
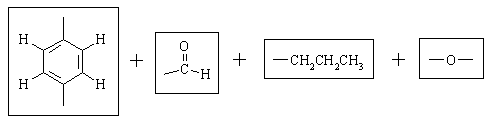
There are only TWO possible ways that these fragments can be put together:
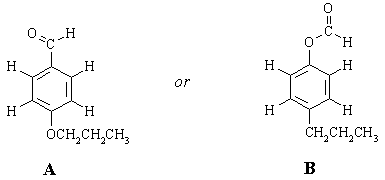
To choose between the two, we need to take a closer look at the nmr spectrum and ask "how will the spectra of these two compounds differ?" One set of protons will have a significantly different chemical shift in the two structures. (Sometimes, more than one set can be identified as being significantly different; sometimes none can, in which case we would need more data). This is the terminal CH2 group in the CH2CH2CH3 chain. In A, it's attached to oxygen and should come at around 4 ppm (look at the table of "representative chemical shifts" in Ege). In B, it's attached to an sp2 carbon and should come at 2-2.5 ppm. Clearly, structure A is the one that fits the spectrum the best.
| Go to: |
Instructions for Printing this Document Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
21nov97; wjl