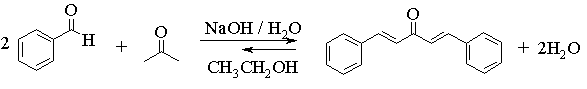
| McMaster University - Chem2OB3 Lab Manual |
Experiment 3. Condensation Reactions of Ketones and Aldehydes. The Aldol Condensation and Formation of Hydrazones.
In the text of this lab you will find several bulleted questions in bold face italics. You are expected to answer these questions or do the assigned reading/work before coming into the laboratory.
References: Brown & Foote, Chapters 15, 18
A. Aldol Condensation
Most of the experiments that you have carried out thus far in this course have involved the manipulation of functional groups. In terms of "real" chemistry as practised in industry or by nature in biological systems, it is important to be able to change the molecular weight of a system by making and/or breaking carbon-carbon bonds. This experiment illustrates one general way this can be done using an aldol reaction. Related chemistry is involved in the reactions of amines (RNH2) or hydrazines (RNHNH2) with ketones and aldehydes. An example of a reaction of this type is the subject of the second part of this lab.
The aldol reaction is a very general and mild method of making carbon-carbon bonds. It is an example of a condensation reaction, which always proceeds in two steps: nucleophilic addition followed by elimination. The aldol reaction involves condensation of two carbonyl compounds, one of which serves as the nucleophile and the other as the electrophile in the first step. The one serving as the nucleophile must have hydrogens on the carbon adjacent to the carbonyl group. As you know, these "a-hydrogens" are rendered somewhat acidic by the C=O group. Removing one by reaction with a base generates an enolate ion, which is a potent carbon nucleophile (much like a Grignard reagent).
The overall transformation that you will carry out is shown in the following equation. Note that this involves making two carbon-carbon bonds and thus two separate aldol reactions.
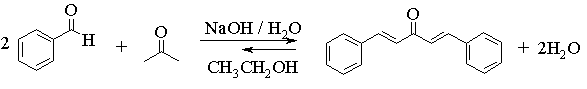
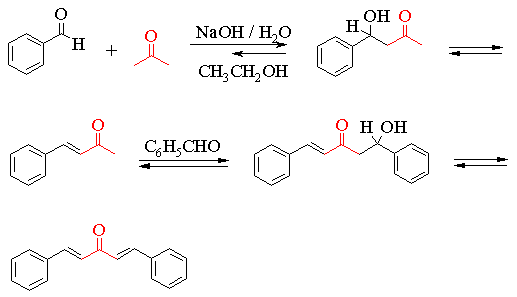
The transformation involves some intermediates which are not shown in the above equation. These are the initially formed b-hydroxy-ketones shown in the scheme below. In some aldol reactions the b-hydroxy-carbonyl compounds are the major products. However, as you will see, all the reactions are reversible and, in the particular case you are studying, the equilibrium constant lies in favour of the unsaturated product.
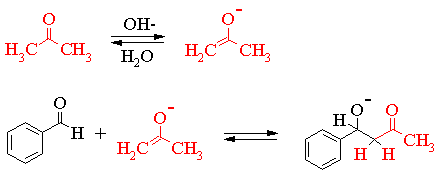
The reaction scheme shown thus far simply records the neutral molecules involved in the transformation. In order to understand what is going on we need to examine some of the ionic intermediates. This is shown in the series of equilibria given on the next page. Please note that resonance structures of the various ionic intermediates are not shown. In order to simplify the mechanism only one half of the reaction is shown.
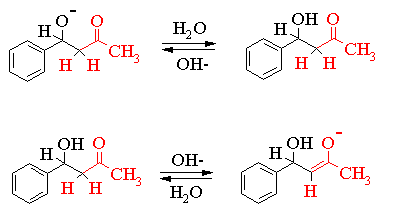
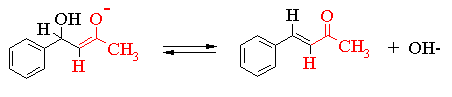
You should also be aware that there are a variety of other reactions occurring simultaneously with the ones shown above.
Procedure
This experiment is conducted using microscale equipment. You should review the procedures for the use of this type of equipment which is contained in the Microscale Techniques section of the lab manual.
It is important in this reaction to follow precisely the instructions on the amount of materials used. Remember that acetone is a very volatile chemical. You should not let the vessel containing acetone sit around before you add the other reagents.
Use a conical vial (3 mL size) fitted with a spin vane and air condenser.
Place in the vial benzaldehyde (294 mg; 280ÁL), acetone (80.4 mg; 100 ÁL) and then the sodium hydroxide solution (1 mL). (The sodium hydroxide solution provided is made up using a mixture of water (57%) and ethanol (43%)). Stir the contents of the vial at room temperature for 30 minutes. You should observe the formation of a yellow precipitate during this reaction period.
Isolate the product by filtering it under vacuum using the Hirsch funnel. Make sure you transfer all of the solid into the funnel. Wash the product with water (several washes with 1 mL portions of distilled water) and check that the filtrate from the final wash is near neutral using indicator paper. If the filtrate is still basic then continue to wash the product until all of the sodium hydroxide is removed. Dry the product by leaving it as a pressed down cake on the Hisrch funnel while you pull air through it using the aspirator.
Purify the product by recrystallization from 95% ethanol using a small beaker or Erlenmeyer flask.
Weigh the recrystallized product, record the weight and calculate the yield obtained in your preparation.
Determine and record the melting point of your product.
Obtain an ir spectrum of your product using the nujol mull method. Paste your spectrum in your lab book and assign the key bands.
B. Formation of Hydrazones
Spectroscopic techniques are becoming the norm for the identification of organic materials. These spectroscopic techniques are normally combined with measurement of key physical properties, such as melting point, of a material. However, it is also very useful to use small scale reactions in order to characterize the functional groups present within a molecule. Where these reactions lead to a solid material the determination of its melting point gives further confirmation of its structure.
One common diagnostic test for the presence of an aldehyde or ketone group within in an organic molecule is to see if it will from a 2,4-dinitrophenylhydrazone. This is a simple test tube reaction that generally leads to the formation of a solid.
The chemistry associated with this reaction is shown below.
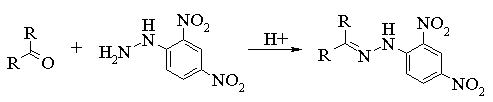
Procedure
Add approximately 50 mg of the product you isolated above to 2 mL of a 0.1M solution of 2,4-dinitrophenylhydrazine contained in a small test tube. (The reagent is dissolved in a mixture of ethanol and phosphoric acid.) Warm the tube for a few minutes, then allow to cool to crystallize. Collect the product by vacuum filtration using the Hirsch funnel. Recrystallize the dinitrophenylhydrazone from hot ethanol. (By this stage in the course you should know how such a recrystallization is done. If you are not sure, then check with your TA.)
Measure the melting point of your 2,4-dinitrophenylhydrazone and record this in your lab book.
| Go to: | Instructions for Printing this Document Chem2OB3 Home Page. |
January 17, 2001