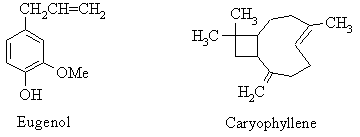
| McMaster University - Chem2OB3 Lab Manual |
Experiment 1a. Essential Oils from Spices: Oil of Cloves or Cinnamon
The technique of steam distillation allows the separation of volatile components from non-volatile materials without the need for raising the temperature of the distillation above 100 oC. It provides a method for the isolation of natural products such as essential oils, which tend to be prone to decomposition at elevated temperatures. Essential oils are volatile compounds responsible for the aromas commonly associated with many plants.
The chief constituents of the essential oils from cloves and cinnamon are volatile aromatic compounds - organic molecules containing one or more benzene rings. Oil of cloves (from Eugenia caryophyllata) is rich in eugenol (4-allyl-2-methoxyphenol; bp 250 oC), which is one of a class of compounds known as phenols, which are compounds containing an hydroxy-substituted benzene ring. Caryophyllene is also present in relatively small amounts, along with other terpenes.
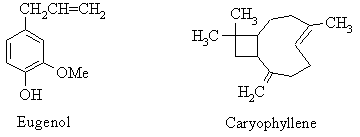
The principal component of cinnamon oil (from Cinnamomum zelyanicum) is cinnamaldehyde (trans-3-phenylpropenal; bp 252 oC), one of a class of natural products known as phenylpropanoids.
In order to complete the distillation in a reasonable length of time, it will be necessary to boil the mixture as rapidly as possible without allowing the boiling mixture to rise above the neck of the Hickman head. This will require that you work with very careful attention during the distillation procedure. The distillation will require one to two hours for either spice. Once the distillation is complete, you may postpone further operations to carry out the preparative gas chromatographic separation of Expt. 1b.
Procedure
Assemble an apparatus using the 10-mL roundbottom vial, a Hickman head, and a water cooled reflux condenser, as shown in the distillation section of the microscale laboratory techniques document. You can decide for yourself whether you need the drying tube shown in the figure. If you are steam distilling cinnamon, insert an air condenser in between the distillation vessel and the Hickman head; you won't need this for oil of cloves. Adjust the temperature of the sand bath initially to about 130 oC. During the distillation, you should be able to increase the sand bath temperature to about 150 oC, as frothing becomes less of a problem.
If you are steam distilling oil of cloves, place about 0.30-g (record actual weight) of ground cloves into the roundbottom vial and add 4.0-mL of water and a boiling stone. For the distillation of cinnamon, put about 6.0-mL of water, then about 0.50-g of ground cinnamon, and finally a small magnetic stirring bar (available from your TA) instead of the boiling stone. In order to prevent the cinnamon from clumping, it will be necessary to stir the mixture during the distillation.
Begin heating the mixture to provide a steady rate of distillation. The temperature of the mixture can be controlled by raising or lowering the roundbottom vial in the sand. You may also push the sand towards the flask with a spatula when a higher temperature is needed, or the temperature of the sand may be increased. With cinnamon, it is essential to control the temperature so that the boiling mixture does not rise above the neck of the Hickman head. While being careful, remember that in order to complete the distillation in a reasonable length of time, you will have to boil the mixture as rapidly as possible without allowing it to splash into the Hickman head. The distillation with cloves can be done with more vigorous boiling. However, some care must be exercised to ensure that the mixture doesn't froth too much.
To perform the following operations, it will be necessary to temporarily remove the reflux condenser. During the distillation, replace the water lost through distillation by adding fresh hot water to the boiling mixture using a Pasteur pipet which is passed through the Hickman head into the roundbottom vial. As the distillation proceeds, collect the distillate with a second Pasteur pipet (5¾ inch) and use it to rinse the walls of the Hickman head at 5-10 minute intervals. As the distillate continues to collect in the reservoir of the Hickman head, use the Pasteur pipet to transfer it to a 5-mL conical vial. The rinsing and transferring operations are best accomplished if the Pasteur pipet is bent slightly at the end.
If you are distilling oil of cloves, continue the steam distillation with frequent rinsing of the Hickman head and collection of the distillate in the 5-mL conical vial until you have obtained 2.5-mL of distillate, then proceed to the next paragraph. If you are distilling cinnamon, continue the steam distillation until you have collected about 1.5-mL of distillate. Raise the assembly and allow the roundbottom vial to cool until you can touch it with your fingers. Remove the flask and discard the mixture of ground cinnamon and water, but save the stirring bar. It is not necessary to clean the roundbottom vial or the Hickman head at this stage. Add 0.50-g of fresh cinnamon (record actual weight), 6.0-mL of water, and return the magnetic stirring bar to the flask. Reassemble the apparatus and continue the distillation. Rinse the Hickman head as before and transfer the distillate to the same 5-mL conical vial. When you have collected another 1.5-mL of distillate (for a total of 3.0-mL), the distillation may be stopped and you may proceed to the next paragraph.
The essential oil will now be extracted with dichloromethane (see Method A of Microscale Extractions). Using a calibrated Pasteur pipet, add 1.0-mL of dichloromethane to the 5-mL conical vial containing the distillate. Cap the vial securely and shake it vigorously with frequent venting. Allow the layers to separate. Using a filter-tip pipet, transfer the lower (dichloromethane) layer to a second dry 5-mL conical vial. Repeat this procedure with two more 1.0-mL portions of dichloromethane, combining all the extracts into the same 5-mL conical vial. If there are visible drops of water in the vial, it will be necessary to transfer the dichloromethane solution with a dry pipet into another dry conical vial. Dry the dichloromethane solution with 3-4 microspatulafuls (measured with the V-groove end) of granular anhydrous sodium sulfate to the conical vial. Let the mixture stand for 10-15 minutes with occasional stirring.
While the organic solution is drying, clean and dry the first 5-mL conical vial and weigh it accurately. With a clean, dry filter-tip pipet, transfer the dried organic layer to this tared vial. Use small amounts of fresh dichloromethane to rinse the solution completely into the tared vial. Be careful to keep the sodium sulfate from being transferred to the vial. Remove the dichloromethane by leaving the vial in the hood until the solvent has evaporated and nothing but an oily residue remains. Alternatively, you may evaporate the dichloromethane in the hood with a gentle stream of dry air while heating the conical vial in a warm (40-50 oC) sand bath. Tap the vial with your finger to provide agitation in order to facilitate removal of the remaining solvent.
When the solvent has been removed, weigh the conical vial. Calculate the weight percentage recovery of the oil from the original amount of spice used.
Obtain the infrared spectrum of the oil as a pure liquid sample. It may be necessary to use a micro syringe or a Pasteur pipet with a narrow tip to transfer a sufficient amount of liquid from the vial to the salt plates. Include the spectrum in your lab-book, along with an assignment of the principal peaks. Rationalize any differences between your spectrum and the literature spectrum of eugenol or cinnamaldehyde. Cut out the appropriate 1H NMR spectrum of eugenol or cinnamaldehyde, and include it in your lab-book as well. Assign the peaks in the spectrum to the various protons in the molecule.
| Go to: | Instructions for Printing this Document Chem2OB3 Home Page. |
23dec99; jp