McMaster University - Chem3D03
| Problem Set #1 | ANSWERS | January 12, 1998 |
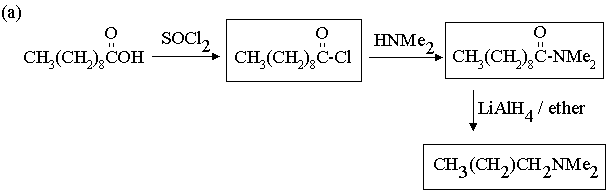
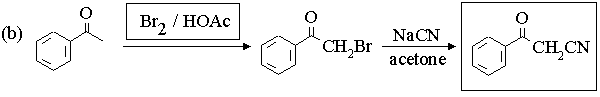
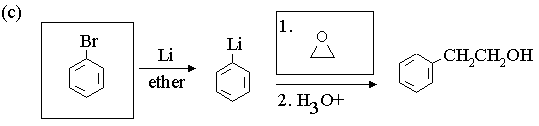
1. Fill in the boxes in the reaction sequences below.

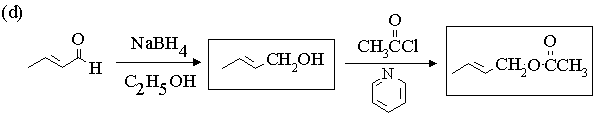
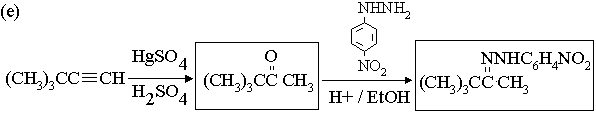
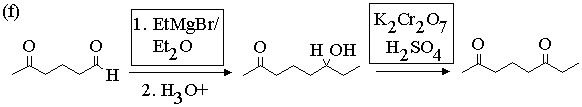
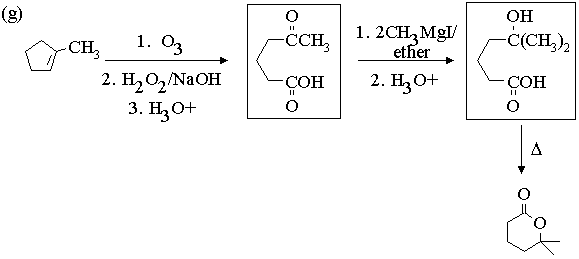
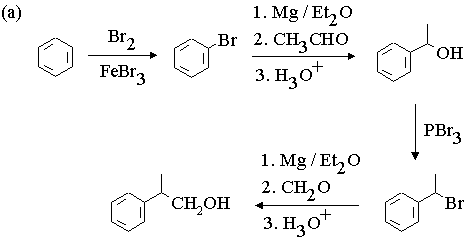
2. Show with equations how the molecule on the left could be converted to the molecule on the right. You may use any other reagents you need. Draw the structures of all intermediate compounds in each sequence. Mechanisms are not required.
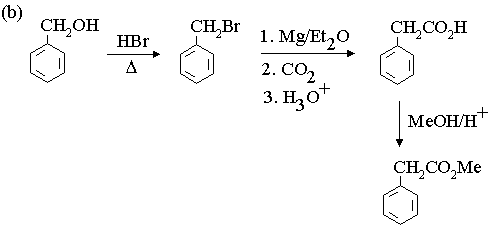
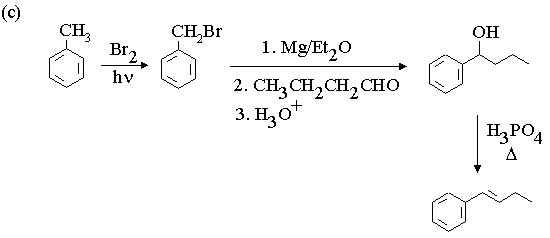
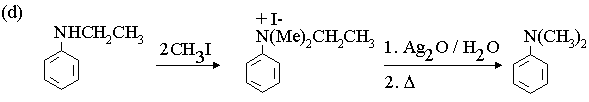
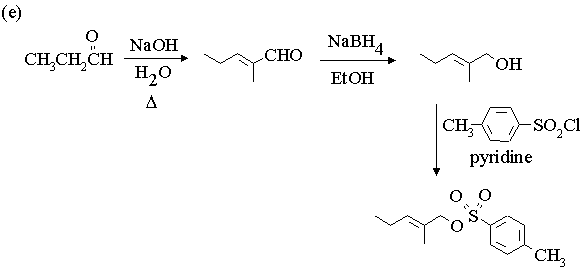
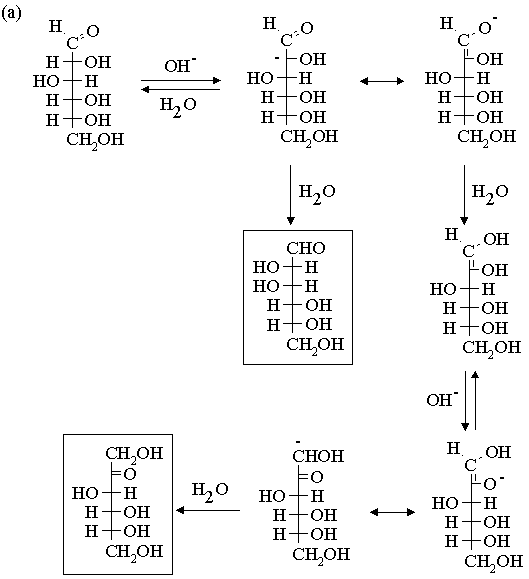
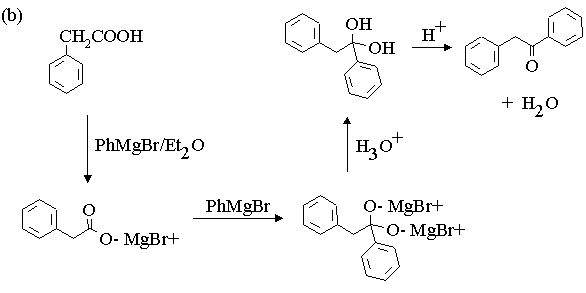
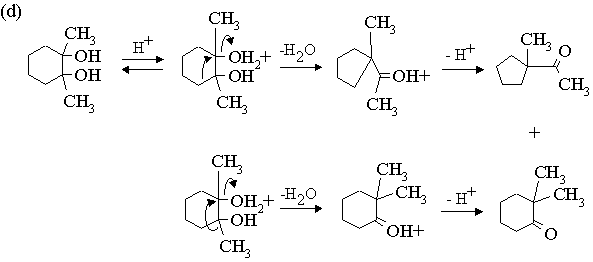
3. Propose mechanisms for the following reactions.
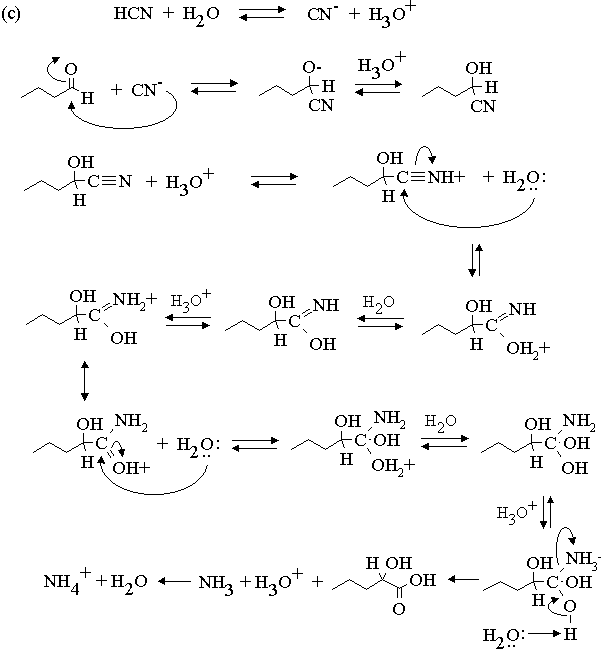
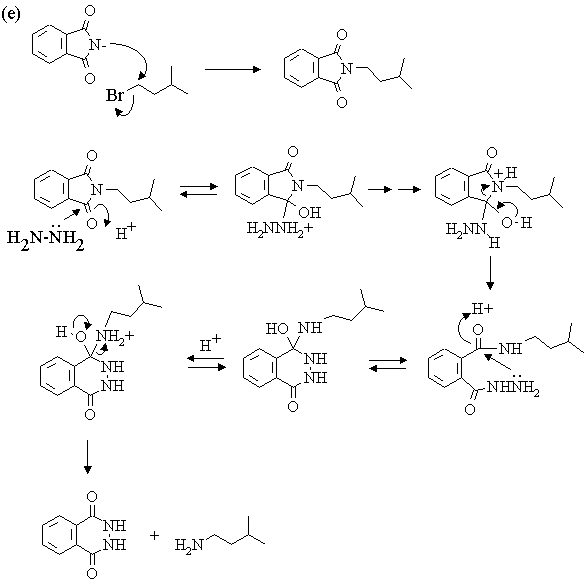
4. The infrared and 1H nmr spectra of a compound with molecular formula C10H12O2 are shown below. Deduce the structure of the compound from these data. Note that in the nmr spectrum, the integration has been done for you; the number of protons responsible for each of the "signals" is indicated right above it in the spectrum.
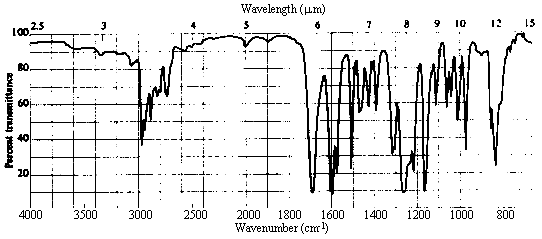
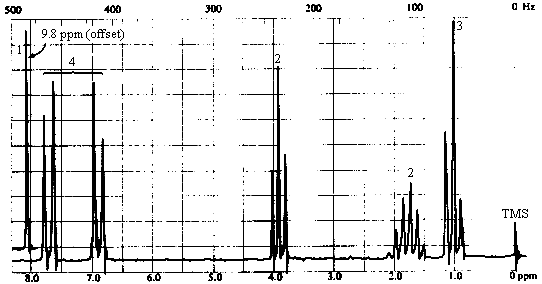
Answer
| 1. Molecular Formula | C10H12O2 -> 5 sites of
unsaturation. For a molecule this size, (i.e., only ten carbons), such a large number of
sites of unsaturation is usually due to the presence of a phenyl ring (which provides 4
sites on its own). For a quick proof, take a quick glance at the 1H nmr
spectrum, and look for signals in the 7-8 ppm (aromatic proton) region. No question,
they're there! This leaves only one site unaccounted for. |
| 2. Infrared Spectrum | - strong peak at ~1700 cm-1 -> C=O (this accounts
for the other site of unsaturation!) - nothing at ~3500 cm-1 -> no OH, so a carboxylic acid or alcohol can be ruled out. - weak absorption in the 3000-3100 cm-1 region indicative of sp2
C-H stretches, in addition to prominent bands in the 2800-3000 cm-1 range due
to sp3 C-H. There is lots more information here, but this is the easiest to
pull out, and all we need for the present. |
| 1H NMR spectrum | - 6 types of H's: - singlet at 9.8 ppm (1H) -> aldehyde or carboxylic acid proton. The ir spectrum rules out a carboxylic acid, so this must be an aldehyde. The absence of coupling indicates that the -CHO group is bonded to an atom with no protons attached to it. - two doublets at ~6.9 and ~7.7 ppm (4H) -> aromatic protons; symmetrical pattern of two doublets suggests a 1,4-disubstituted benzene. Each doublet corresponds to 2 equivalent protons, each of which has one nearest neighbour:
Note that the two doublets are skewed towards one another and that the magnitude of the splitting is the same for both - this verifies that the two doublets "belong together"; i.e., they represent a pair of protons coupled to each other. We've accomplished alot; check the molecular formula to see what's left! We've accounted for a -CHO group and a -C6H4-. All that's left is three carbons bearing 7 hydrogens and an oxygen. Since all sites of unsaturation are accounted for, the seven H's must all be bound to sp3 carbons. - triplet at ~4 ppm (2H) -> two equivalent protons with 2 nearest neighbours. With only 3 carbons left, this must be due to a CH2 attached to a CH2! - triplet at 1 ppm (3H) -> three equivalent protons with 2 nearest neighbours. This must be due to a methyl group attached to a CH2. The last two bits of information, coupled with the fact that we have only C3H7 left, are only consistent with one structure - CH2CH2CH3! - sextet at ~1.8 ppm (2H) -> two equivalent protons with 5 nearest
neighbours. This is the middle CH2 in the -CH2CH2CH3
chain. |
Let's take stock of what we have:
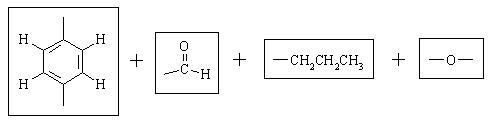
There are only TWO possible ways that these fragments can be put together:
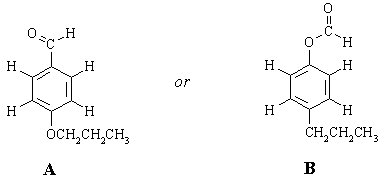
To choose between the two, we need to take a closer look at the nmr spectrum and ask "how will the spectra of these two compounds differ?" One set of protons will have a significantly different chemical shift in the two structures. (Sometimes, more than one set can be identified as being significantly different; sometimes none can, in which case we would need more data). This is the terminal CH2 group in the CH2CH2CH3 chain. In A, it's attached to oxygen and should come at around 4 ppm. In B, it's attached to an sp2 carbon and should come at 2-2.5 ppm. Clearly, structure A is the one that fits the spectrum the best.
5. The highest molecular weight ions in the mass spectrum of an unknown compound arise at 150 and 152 amu, in the intensity ratio 3:1. Its infrared and 1H nmr spectra are shown below. Deduce the structure of the compound from these data.
Answer
Mass Spectrum
The highest molecular weight ion usually corresponds to the molecular ion, and
hence gives the molecular weight of the compound. A pair of peaks separated by 2 amu with
an intensity ratio of 3:1 indicates that the molecule contains a single chlorine atom.
This would normally be verified by looking elsewhere in the spectrum for similar pairs of
peaks representing Cl-containing fragment ions. Thus, the true molecular weight of the
compound R-Cl is 150.5. The fragment R will have a weight of 115 amu, and thus
contains zero or an even number of nitrogen atoms. Without the full mass spectrum, this is
all we can use for now.
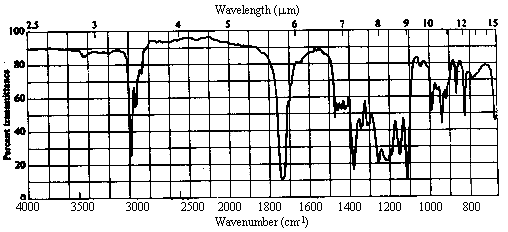
Infrared Spectrum
The presence of a strong peak at ~1730 cm-1 clearly indicates that the molecule
contains a carbonyl group. There are no OH, NH or vinylic C-H absorptions present.
Aldehydes always show a pair of peaks at ~2700 and ~2900 cm-1; while the 2900
cm-1 peak is often difficult to pick out, there is clearly nothing at ~2700.
Thus, the molecule is an aliphatic ketone, ester, or acid chloride (a diamide
can be ruled out simply because the weight of the fragment R is too low). Esters typically
show a strong absorption at ~1200 cm-1 due to the C-O stretch. There is
absorption here, but it overlaps with a number of other bands and is difficult to assign
reliably.
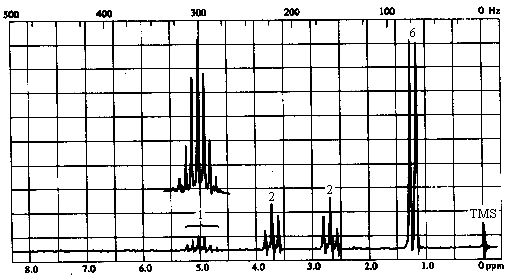
1H NMR Spectrum
The presence of the 1H septet at ~ 5ppm indicates that the two peaks at ~1.2 ppm are a
doublet rather than two singlets; thus, the molecule contains an isopropyl group and a
total of 4 types of hydrogens in relative intensities of 1:2:2:6. Thus, the fragment R
contains two CH3's, two CH2's, and one CH group in addition to the
C=O.
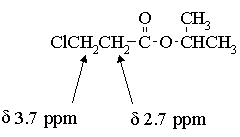
Before going further, let's return briefly to the mass spec data and decide on a molecular formula. C6H11O totals 99 amu; the remaining 16 amu must be due to an oxygen atom. We can conclude that the molecule is an ester of formula C6H11O2Cl.
The chemical shift of the isopropyl CH proton and the fact that the
signal is not split further suggest that we have an isopropyl ester:
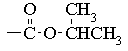
The two 2H triplets at 2.7 & 3.7 ppm indicates that the remaining C2H4Cl
must be arranged as -CH2CH2Cl. Thus, the structure of the molecule
is:
| Go to: | Instructions for Printing this Document Chem3D3 Problem Sets & Answers Chem3D3 Home Page. |
12jan98