Adam Elwi - University of Alberta
Placement: University of Toronto
Supervisor: Dr. Bob McClelland
The Reaction of Quinone Methides with Deoxyguanosine
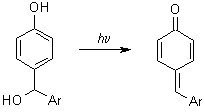
Quinone methides are unsaturated cyclic conjugated ketones involved in a variety of chemical and biochemical reactions. They have been identified as components of plant pigments, have found usage in medicine, and are also intermediates in the metabolism of natural products and drugs. As intermediates in metabolism, quinone methides have received significant attention as being possible carcinogens. In our research, we are investigating the reaction kinetics of several quinone methides with deoxyguanosine, a DNA monomer. We generate the quinone methides photochemically from the corresponding hydroxybenzyl alcohol.

The structure of the quinone methide gives it a high reactivity with a nucleophilic center at the oxygen atom and an electrophilic center at the a carbon atom of the methylene group. As such they are involved in electrophilic and nucleophilic 1,6-addition. By comparing substituent effects at the methylene group and studying the pH rate profiles we are able construct rate equations governing the reactions of these compounds. The pH rate profiles of the following compounds are being studied.
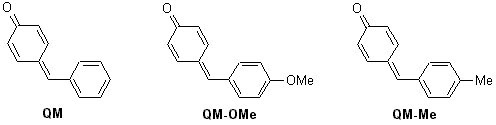
Currently we are studying the pH rate profile of QM with deoxyguanosine and N-methyl guanosine. We also anticipate performing an isodesmic calculation on the protonation of QM, and synthesizing a quinone methide that is stable in solid form and soluble in water so the kinetics with DNA can be studied.
| Back to: | 2000 RISE Scholars RISE Home Page. |
15jul00-wjl