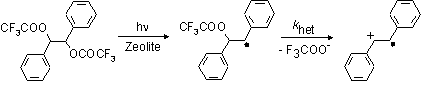
Todd Melville - University of Waterloo
Placement: Dalhousie University
Zeolites are special. They are aluminosilicates with an open framework structure. Zeolites
are used in industry as heterogeneous catalysts for such things as cracking of
hydrocarbons to produce gasoline and cation-exchangers for water softeners. The
framework consists of an interconnected three-dimensional lattice of pores, channels,
and cages made up of corner-sharing SiO4 and AlO4 tetrahedra. The stoichiometry is thus
TO2 and, since aluminum is trivalent, a charge-balancing cation exists beside each
aluminum in the framework to maintain neutrality. This cation is typically an alkali
metal or an alkaline earth metal. The aluminum in the cages act as reactive 'sites'
that can catalyze reactions.
My project involves examining how various zeolites effect organic reactions. First, an
organic 'probe' molecule is incorporated into the zeolite's cage. Using a Nd-Yag laser
we rapidly generate the radical intermediate by photohomolysis of the C-O bond. Then
we can directly observe the rate of ß-heterolysis of the radical, which leads to the
formation of the radical cation of stilbene, using time-resolved diffuse reflectance:
Supervisor: Dr. Fran Cozens
Laser Flash Photolysis of Organic Molecules in Zeolites
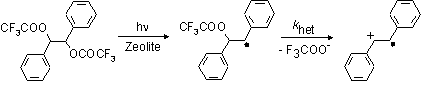
By using different types of zeolites, which vary in their size, shape and/or counterion, and observing the rate constant, k-het, for the ß-heterolysis we hope to gain insight into the nature of the catalytic sites inside the zeolite's cavities and develop a more complete understanding of their potential as solid-state catalysts.
24-jul-96