Sinisa Vukovic - University of Saskatchewan
Placement: University of Toronto
Supervisor: Dr. Bob McClelland
Dynamics of the reactions of carbocations with 2-deoxyguanosine and DNA
Many carcinogens metabolize to species that ionize to carbocation, for example, PAH’s to diol epoxides to benzyl cations and the drug tamoxifen to an acetate ester to a allylic carbocation. Carbocations cause mutations by covalently binding to DNA, mainly at guanine and adenine. My research is aimed at studying model carbocations, using a combination of product analysis and LFP.
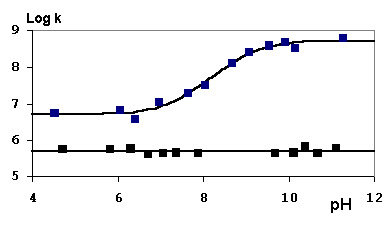
I have been looking at four systems, where the cation is obtained either by ground or excited state SN1 solvolysis from the corresponding acetate. 2-Deoxyguanosine (dG) does not effectively quench these cations at pH 7 but to our surprise very effectively quenches at pH 9. Results of laser flash photolysis are shown in the Figure below. In the case of the bis-(p-methoxyphenyl) system, the major product has been identified by NMR and mass spectrometry.

Experiments with DNA are in progress. DNA appears to be a better quencher at pH 7 than dG. There is also the possibility of saturation kinetics indicating non-covalent binding prior to the actual chemical reaction.
| Back to: | 1999 RISE Scholars RISE Home Page. |
08-sep-99