| McMaster University - Chem2O06 Lab Manual | 1997/98 |
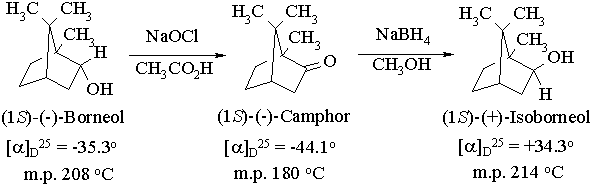
Experiment 7. Isomerization of an Alcohol by Oxidation-Reduction: Borneol, Camphor, and Isoborneol
References: Ege, Chapter 10,12,13; Microscale Techniques
In this experiment you will convert a chiral alcohol into its chiral diastereomer using a scheme involving oxidation to the ketone followed by stereoselective reduction to the diastereomer using sodium borohydride:

The first part of this experiment employs an oxidizing agent which has not been covered in lectures - hypochlorous acid. While it is less commonly used than dichromate, it is much less expensive (we make it by treating sodium hypochlorite (household bleach) with acetic acid) and much less toxic. In the second part, we will employ sodium borohydride to reduce the ketone obtained in the first step back to an alcohol. In camphor, however, the top and bottom faces of the carbonyl group are different, and we will attempt to take advantage of this to selectively reduce the ketone to the other diastereomer of the alcohol. The degree to which we are successful in carrying out this stereoselective reaction will be determined using gas chromatography.
In addition to providing some interesting chemistry, this experiment will introduce the student to microscale laboratory techniques, which are used for handling very small quantities of materials. While the basic principles and operations are the same as those in the macroscale experiments that have been used so far in the course, the techniques and equipment employed for sample manipulation, reactions, extractions, distillation, recrystallization, etc., are very different. You will need a special microscale kit, which you will obtain from your TA at the beginning of the lab period and return at the end. Be sure to read the section on Microscale Techniques, paying particular attention to the specific sections dealing with:
Oxidation of Borneol with Sodium Hypochlorite
Aqueous sodium hypochlorite (NaOCl), or common household bleach, can be used to oxidize secondary alcohols to ketones. The reaction occurs more rapidly under acidic conditions, so it is thought (we're not actually sure) that the actual oxidizing agent is hypochlorous acid (HOCl), generated by the acid base reaction between sodium hypochlorite and acetic acid. You have seen this reagent before, as that employed for the synthesis of a-chloro alcohols from alkenes (the chlorine analog of the reaction of alkenes with bromine in water). In alcohol oxidations, the reaction probably proceeds via E2 elimination of the alkyl hypochlorite produced by reaction of the initial alcohol with HOCl:
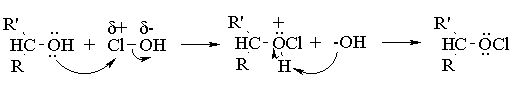
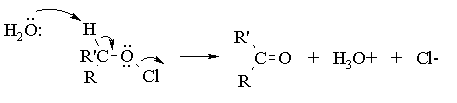
To ensure that complete oxidation of the alcohol occurs, it is necessary to employ an excess of sodium hypochlorite. Since the concentration of sodium hypochlorite in bleach can vary, a test for excess hypochlorite is performed by using starch-iodide indicator paper. In acidic solution, iodide is oxidized by hypochlorite to iodine:
![]()
The iodine then reacts with the starch in the test paper to produce the characteristic purple colour of the starch-iodine complex.
Reduction of Camphor with Sodium Borohydride
Metal hydrides of the Group III elements such as lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) are widely used for the reduction of carbonyl groups. Both reagents are ready sources of hydride ion (H:-), a very strong base and when associated with aluminum or boron, a good nucleophile. Lithium aluminum hydride is extremely reactive, and reduces the C=O group in aldehydes, ketones, carboxylic acids, esters, and amides. Sodium borohydride is a much milder reducing agent, working effectively only with aldehydes and ketones. The reduced reactivity of NaBH4 allows it to be used even in alcohol or aqueous solvents, whereas LiAlH4 reacts violently with these solvents to produce hydrogen gas. Hence, it can only be used in anhydrous, nonhydroxylic solvents like THF or diethyl ether. In the present experiment, sodium borohydride is used because it is easily handled and the results of reductions using either of the two reagents are essentially the same. The mechanism of sodium borohydride reduction of a ketone or aldehyde is very similar to that for hydroboration of a C=C bond:
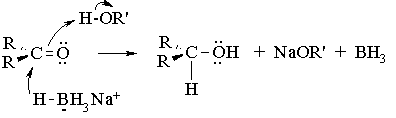
Note that all four hydrogen atoms in the borohydride reagent are available as hydrides (H:-), and thus one equivalent of borohydride can reduce four equivalents of ketone. All the steps are irreversible. Usually, excess borohydride is used because the purity of the material is uncertain.
In principle, the reduction of camphor can give two diastereomeric alcohols, corresponding to reaction of borohydride at the two faces of the C=O bond. Reaction at the top ("exo") face regenerates the original starting material, borneol. Reaction at the bottom ("endo") face yields the other diastereomer, isoborneol:
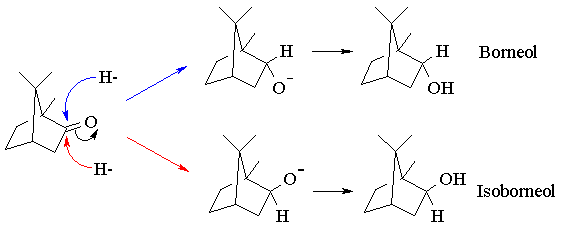
Inspection of a molecular model of camphor will reveal that the exo face is considerably more sterically hindered than the endo face, due to the presence of one of the geminal methyl groups. Since "endo-attack" of borohydride avoids this steric interaction, we might predict that it should proceed faster than exo-attack, leading to stereoselective reduction. The use of steric effects to control reactivity in this way is termed steric approach control. The extent to which we are successful in controlling the stereoselectivity of the reduction will be gauged by determining the relative yields of borneol and isoborneol using gas chromatography.
Procedure: Oxidation of Borneol
Procedure: Reduction of Camphor
| Go to: | Instructions for Printing this Document Chem2O06 Home Page. |
15dec97; wjl