| McMaster University - Chem2O06 Lab Manual | 1997/98 |
Experiment 7. Part B: Reduction of Camphor.
Reweigh the 10 mL Erlenmeyer flask from Part A to determine the weight of camphor remaining. If the amount is less than 0.100 g, obtain some camphor from the supply shelf to supplement your yield. If it is more than 0.100 g, scale up the reagents accordingly from the amounts given below.
To the camphor in the 10 mL flask (0.100 g), add methanol (0.5 mL). Stir with a glass rod or microspatula until the solid has dissolved. In portions, cautiously and intermittently add 0.060 g of sodium borohydride to the solution. If necessary, cool the flask in an ice bath to keep the reaction mixture at room temperature. When all the borohydride is added, boil the contents of the flask on a steam bath at 70 oC for two minutes.
Allow the reaction mixture to cool for a few minutes, and then carefully add ice water (3.5 mL). Collect the white solid which forms by filtering through a Hirsch funnel, and suction-dry it for several minutes while you clean and dry the 10 mL Erlenmeyer flask. Transfer the solid back to the clean flask, and add ether (4.5 mL) to dissolve the solid followed by 3-4 microspatulafuls of anhydrous sodium or magnesium sulfate. Filter the mixture with a filtering pipet into a tared 25 mL Erlenmeyer flask. Rinse the 10 mL flask with a further 1.0 mL of ether and filter this into the 25 mL flask. Evaporate the solvent in the hood, again being careful not to overheat past dryness in order to avoid loss of the product due to sublimation.
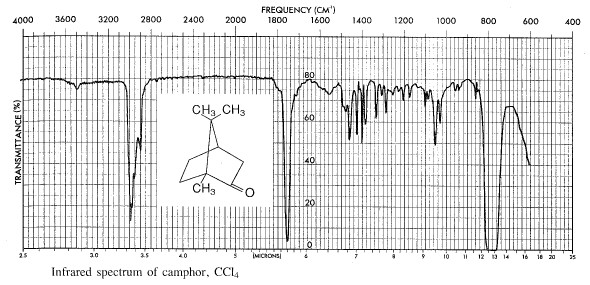
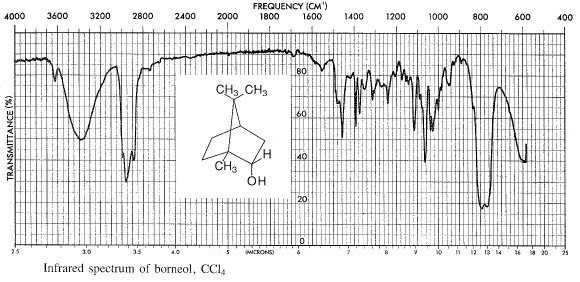
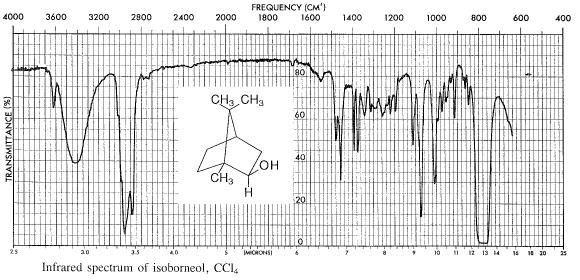
Determine the weight of the product and calculate the percentage yield. Determine the melting point in a sealed capillary tube. Determine the infrared spectrum of the product using the method given in part A.
Your TA will organize a time for you to obtain a gas chromatogram of your product. Review the section on gas chromatography in Expt 4A. Take a small amount of the product and dilute it with 1-3 mL of ether immediately before glc analysis.
You should record the instrument parameters carefully: i.e. the type of column used, column temperature, the Helium flow rate, chart paper movement rate in cm/min., and the instrument manufacturer's name. All these data should be recorded on the chart paper (including your name, the date, and what you injected onto the column!) as well as in your Lab Notebook. Work out the retention time Rt for each of your peaks and label them, including the solvent peak. Measure the relative areas of the peaks due to borneol and isoborneol and report their relative yields, assuming that the weight ratio of the components is equal to the ratio of the area of the peaks.

| Go to: | Instructions for Printing this Document Experiment 7 - Main Page Part A: Oxidation of Borneol Chem2O06 Home Page. |
15dec97; wjl