Chem2O6
- 1997/98| Problem Set #1 | ANSWERS | September 18, 1997 |
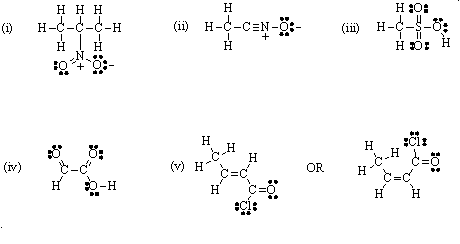
1. Write the following condensed formulas as Lewis structures:
(i) (CH3)2CHNO2 (ii) CH3CNO (iii) CH3SO3H (iv) CHOCO2H (v) CH3CHCHCOCl
Answer:

2. (a) Which of the following species would you expect boron trifluoride (BF3) to form a complex with?
(CH3)2CHCH2CH3 CH3CH2OCH3 AlCl3 CH3NHCH3 CH3F
(b) Draw the Lewis structure of the complex.
(c) Which complex would you expect to be the most stable? Why?
Answer:
(a) CH3CH2OCH3, CH3NHCH3, and CH3F because these three compounds have atoms with lone pairs of electrons. 2-Methylbutane has no lone pairs, while AlCl3 is electron deficient. In fact, it is itself a Lewis acid like BF3 and exists as the dimer (Al2Cl6) rather than the monomer.
(b)
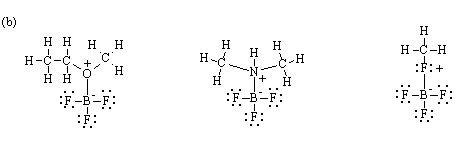
(c) The complex with dimethylamine should be the more stable because N is less electronegative than O and F, and is better able to donate its lone pair of electrons to the Lewis acid. The actual order of stability would be N > O > F.
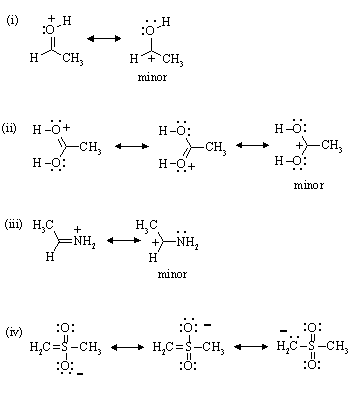
3. Write resonance structures for the following, noting any structures which might be minor contributors.
(i) [CH3CHOH]+ (ii) [CH3CO2H2]+ (iii) [CH3CHNH2]+ (iv) [CH2SO2CH3]-
Answer:
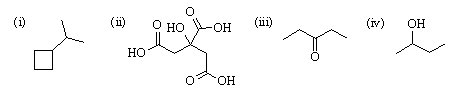
4. Give line structures for:
(i) isopropylcyclobutane
(ii) citric acid
(iii) 3-pentanone
(iv) 2-butanol
Answer:
5. Complete the following reactions, indicating (where asked) the structure of any intermediates. Where appropriate, give the stereochemistry of the products.
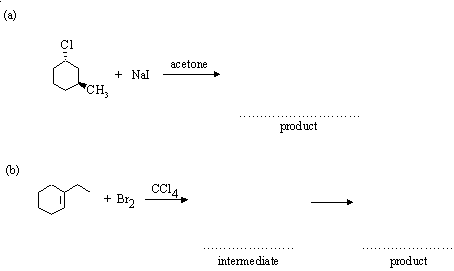
Answer:
(a) This is an SN2 substitution reaction. The nucleophile attacks the electrophilic carbon of the alkyl halide from the rear, causing loss of the leaving group and inversion of configuration:
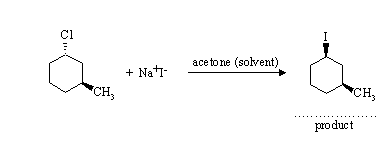
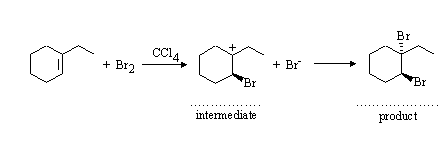
(b) Electrophilic addition of Br2 to an alkene. The initial step involves addition of "Br+" to yield the more stable carbenium ion intermediate. The intermediate reacts with Br- from the least hindered side, for overall trans-addition across the double bond.
| Go to: | Instructions for Printing this Document Problem Set 1 Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
18sep97; wjl