Chem2O6
- 1997/98| Problem Set #1 | September 11, 1997 |
1. Write the following condensed formulas as Lewis structures:
(i) (CH3)2CHNO2 (ii) CH3CNO (iii) CH3SO3H (iv) CHOCO2H (v) CH3CHCHCOCl
2. (a) Which of the following molecules would you expect boron trifluoride (BF3) to form a complex with?
(CH3)2CHCH2CH3 CH3CH2OCH3 AlCl3 CH3NHCH3 CH3F
(b) Draw the Lewis structure of the complex.
(c) Which complex would you expect to be the most stable? Why?
3. Write resonance structures for the following, noting any structures which might be minor contributors.
(i) [CH3CHOH]+ (ii) [CH3CO2H2]+ (iii) [CH3CHNH2]+ (iv) [CH2SO2CH3]-
4. Draw line structures for:
(i) isopropylcyclobutane
(ii) citric acid
(iii) 3-pentanone
(iv) 2-butanol
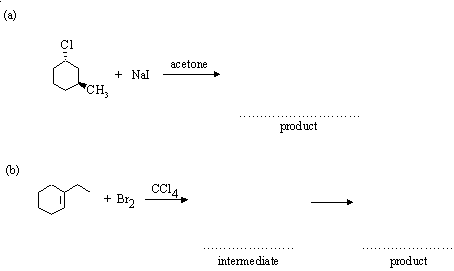
5. Complete the following reactions, indicating (where asked) the structure of any intermediates. Where appropriate, give the stereochemistry of the products.

| Go to: | Instructions for Printing this Document Answers Chem2O6 Problem Sets & Answers Chem2O6 Home Page. |
11sep97