The more stable of two products from a given reaction is not necessarily formed more rapidly than the less stable one. Consider the equations and free energy diagrams below.
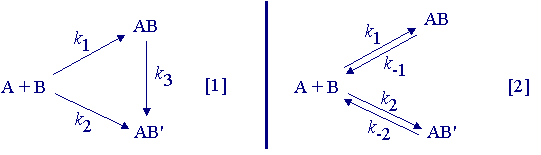
Two products AB and AB' can arise from the reaction of A with B either directly (k1 ~ k2 >> k3) or indirectly (k1 >> k2) by rearrangement of AB to AB' (eq. 1). Alternatively, AB and AB' may be interconverted via A and B because of reversibility (eq. 2). The rates of formation of AB and AB' are determined by the activation energies for the rate-determining steps.
If ![]() G1
G1![]() <
< ![]() G2
G2![]() , then AB (the less stable product) will be formed fastest. If
, then AB (the less stable product) will be formed fastest. If ![]() G3
G3![]() is larger than
is larger than
![]() G1
G1![]() , AB will be isolated from reactions carried out at low temperature or for short time periods. If AB is isolated, the reaction is said to be running under kinetic control.
, AB will be isolated from reactions carried out at low temperature or for short time periods. If AB is isolated, the reaction is said to be running under kinetic control.
If the reaction is left for a long time or if the temperature is high, AB will be converted to AB', either by a pathway connecting AB and AB' with a rate determined by ![]() G3
G3![]() or, if that barrier is very high, by reverting to starting materials (barrier
or, if that barrier is very high, by reverting to starting materials (barrier ![]() G1
G1![]() ) and passage over barrier
) and passage over barrier ![]() G2
G2![]() . When AB' is isolated, the reaction is said to be under thermodynamic control.
. When AB' is isolated, the reaction is said to be under thermodynamic control.
An example of a system in which either kinetic or thermodynamic control can be selected is the following.
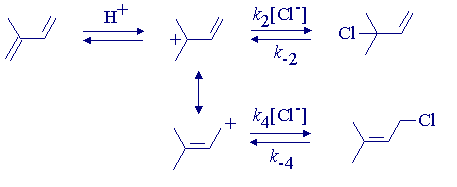
The allylic cation is captured most rapidly at the tertiary center to give 3-chloro-3-methyl-l-butene as the kinetic product at -10o. After a long time at -10o, the more stable primary chloride becomes the major product.
Another type of system which can exhibit this type of behaviour is one in which two different substrates compete in a reversible reaction with another substrate. An example of this is semicarbazone formation, which is the reaction that will be examined in this experiment. The formation of semicarbazones by acid-catalyzed condensation of a carbonyl compound with semicarbazide is a reversible process. If two carbonyl compounds compete for a limited amount of semicarbazide, it is sometimes possible to isolate the kinetic mixture of semicarbazones before equilibrium is established. Given time, the mixture moves toward the equilibrium position, giving the thermodynamic product composition.

Procedures
(i) Preparation of Cyclohexanone semicarbazone & Furfural semicarbazone.Semicarbazide hydrochloride (2 g) and sodium acetate (4 g) are added to 8 mL of methanol. The large crystals are ground with a glass rod. Finely divided sodium chloride separates.
Divide this solution into two equal portions. To one add 1.0 mL of cyclohexanone and to the other add 1.0 mL of furfural (ensure that it has been freshly distilled). Allow these mixtures to stand for about 15 minutes. The products are obtained by addition of a small amount of water, in which they are insoluble. Filter, and then wash the crystals with water to remove HOAc, AcO- and traces of semicarbazide. Recrystallize from ethanol or methanol, using water if necessary.
Record the melting point of each of your semicarbazones. Cyclohexanone semicarbazone is reported to melt at 166 oC; furfural semicarbazone at 202 oC
(ii) Competitive Semicarbazone Formation
Prepare a homogeneous solution of cyclohexanone (0.05 mole, 4.9 g) in furfural (0.05 mole, 4.9 g, freshly distilled) and divide it into two parts in the ratio 2:1. Cool the large sample in an ice bath.
Prepare a second solution by dissolving semicarbazide hydrochloride (0.025 mole, 2.8 g) and sodium acetate (0.05 mole, 4.1 g) in 30 mL of a 2:1 mixture (by volume) of water / 95% ethanol. Divide this solution into two parts (2:1), and cool the large one in the ice bath. Set up a filter for vacuum filtration before proceeding to the next step.
Mix the two warm solutions, insert a magnetic stirrer and stir for one hour. Mix the two cold solutions by pouring the carbonyl compounds into the semicarbazide reagent, note the time, and mix them well with a spatula. Keep stirring and scratching the mixture, while cooling, for 2 -3 minutes (record the exact actual time). Filter off the crystals rapidly and wash them on the filter with cold water. When the material is dry, weigh it and take the melting point. Estimate the composition by using m.p. data. You can use a Raoult's Law expression to do this:
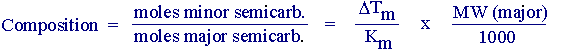
where
Km = molal melting point depression constant (assumed to be 8 oC for each semicarbazone).
![]() Tm = observed melting point depression (in oC) relative to major semicarbazone.
Tm = observed melting point depression (in oC) relative to major semicarbazone.
Also, calculate the composition on a mole percent basis (% minor semicarbazone). When the room temperature reaction has run for the allotted time, isolate the product and estimate the composition using the same procedure as above.
(iii) UV Analysis.
You will be given (on a separate sheet) the ultraviolet absorption spectra of the semicarbazones of cyclohexanone and furfural. Prepare solutions of the mixed semicarbazones obtained from your hot and cold reactions in 95% ethanol. The solutions need not be prepared with analytical accuracy (since you have been given ![]() ), but you should ensure that your concentrations are such that the final absorbance will be in the 0.5 - 2.0 range. Use the Beer's Law expression to calculate the ratio of semicarbazones present (l = 1 cm for the cells you will use).
), but you should ensure that your concentrations are such that the final absorbance will be in the 0.5 - 2.0 range. Use the Beer's Law expression to calculate the ratio of semicarbazones present (l = 1 cm for the cells you will use).
(iv) NMR Analysis.
You will be given 1H nmr spectra of both pure semicarbazones and the hot and cold reaction products. In your lab report, assign the signals to the appropriate protons in each semicarbazone. Can you use the "hot" and "cold" spectra to make quantitative deductions? Why not?
Questions
1. Write the complete mechanism for the formation of a semicarbazone from reaction of a ketone or aldehyde with semicarbazide in aqueous solution.2. Which semicarbazone is formed faster? Is there any structural difference that could account for the faster rate of reaction of this carbonyl compound with semicarbazide?
3. Which semicarbazone is most stable? How can you rationalize its greater formation constant,
Kf = {[Semicarbazone][H2O]} / {[carbonyl cpd][semicarbazide]}
(Consider not only structural effects in the isolated molecules but also possible intermolecular forces in the crystals.)
4. What must be the pH, approximately, of the medium in which the competitive semicarbazone formation took place?
5. Which analysis do you consider to be the most reliable and why?