Aldehydes and ketones are extremely versatile compounds for organic synthesis, acting either as electrophiles (i.e. nucleophilic addition) or as nucleophiles (i.e. enol/enolate reactions). Because of this versatile reactivity, synthetic sequences involving polyfunctional molecules frequently require that aldehyde and/or ketone carbonyl groups be protected in order to stop undesirable side reactions. The protecting group is then removed at a later stage in the synthesis. The most common protecting group for aldehydes and ketones is the ethylene acetal or ketal (1,3-dioxolane derivative), which is easily prepared from the carbonyl compound and ethylene glycol in the presence of an acid catalyst. As you know, acetal/ketal formation is fully reversible under acidic conditions; the equilibrium is driven to the desired ketal by offsetting the equilibrium by removal of water using azeotropic distillation. The carbonyl compound is regenerated by simply mixing the acetal/ketal with water in the presence of trace acid.
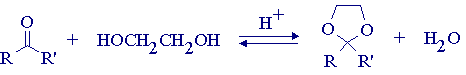
In this experiment, an a,b-unsaturated ketone will be synthesized by a route whose key step is a Grignard addition to the ester group in ethyl acetoacetate, a b-ketoester. The first step is protection of the ketone carbonyl as the ethylene ketal (1); the last step is an acid-catalysed dehydration which occurs during deprotection of the ketone. The final product (3) will be purified by column chromatography.
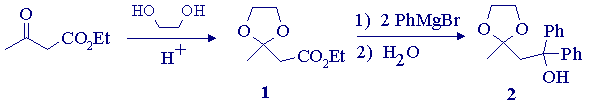
(i) Synthesis of Protected b-Ketoester (1)
In a 100-mL round bottomed flask, add ethyl acetoacetate (0.1 mol), ethylene glycol (0.105 mol), toluene (50-mL), a trace (0.05 g) of 4-toluenesulfonic acid monohydrate, and 3-4 boiling chips. Attach a Dean-Stark trap, reflux condenser and heating mantle, wrap the flask up to the condenser with glass wool, and heat the flask so that the toluene refluxes vigorously. Reflux the mixture until no more water collects in the trap (about 45 minutes). Cool the mixture to room temperature, transfer it to a separatory funnel, and wash the solution with 5% aqueous sodium hydroxide (15-mL) followed by water (2 X 20-mL). Dry the organic layer over anhydrous potassium carbonate, filter, and remove the solvent on the rotary evaporator.* Transfer the residue to a 25-mL round bottomed flask and distil it under reduced pressure (aspirator), collecting the fraction boiling at ~110oC (30 mmHg). Record the exact bp (and pressure), yield, and IR spectrum (as a neat film) of your product. Record the IR (film) spectrum of ethyl acetoacetate for comparison.
(ii) Synthesis and Reaction of Phenylmagnesium bromide with the protected b-ketoester.
In a fume hood, set up a 2- or 3-neck 100-mL roundbottom flask with an addition funnel, magnetic stirrer and reflux condenser fitted with a calcium chloride drying tube. Flame-dry the complete apparatus with a bunsen burner, being sure to first remove any volatile materials from the vicinity. When the apparatus is cool to the touch, add magnesium turnings (0.065 mol), anhydrous diethyl ether (10-mL), and a tiny crystal of iodine to the flask. Place bromobenzene (0.06 mol) and anhydrous ether (10-mL) in the addition funnel, and add a few drops of this solution to the magnesium. Start the stirrer, and wait until the formation of the Grignard reaction commences (this will be evident when the ether starts to reflux and the solution takes on a gray-brown colour). Add another 20-mL portion of anhydrous ether to the bromobenzene solution in the addition funnel, and add the solution dropwise at such a rate so as to maintain a gentle reflux. After the addition is complete, reflux the mixture with stirring on a hot water bath for about 10 minutes. Cool the flask in an ice bath, and then add a solution of ethyl acetoacetate ethylene ketal (1); 0.025 mol) in anhydrous ether (10-mL) dropwise through the addition funnel. After the addition is complete, stir the mixture for a further 20-minutes at room temperature, and then add ice water (20-mL) to the flask. When the ice has melted, add a further amount of ether (10-mL), and (patiently) stir the mixture until the gummy solid dissolves, adding more ether as required. Transfer the mixture to a separatory funnel, and separate the layers. Extract the aqueous layer with ether (2 X 10-mL), combine the ether layers, wash them with water (10-mL), and dry over anhydrous magnesium sulfate.* Filter and evaporate the solvent on the rotary evaporator. The yellow-orange oil obtained should crystallize on cooling.*
Recrystallize the crude product from petroleum ether. Record the yield, mp, and the IR spectrum as a KBr pellet.
* may be left at this stage.
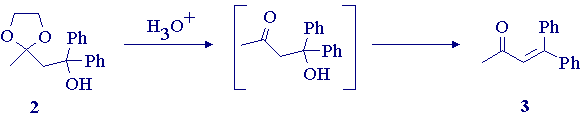
(iii) Hydrolysis & Dehydration of Ketal 2.
Place the ketal 2 (.010 mol), concentrated hydrochloric acid (1-mL), acetone (25-mL), water (1.5-mL), and 3-4 boiling chips in a 50-mL round bottomed flask fitted with a reflux condenser. Reflux the mixture on the steam bath for one hour. Transfer the cooled mixture to a separatory funnel, add water (25-mL), and extract with ether (2 X 15-mL). Combine the ether layers, wash them with saturated aqueous sodium bicarbonate (15-mL) and water (15-mL), and then dry over anhydrous magnesium sulfate. Filter and evaporate the solvent on the rotary evaporator. Record the yield of the crude product. Purify by column chromatography on silica gel using toluene as the eluant. Record the yield and IR spectrum (in CCl4) of your purified product.
Questions
1. Write the mechanisms for formation of ketal 1 and hydrolysis of ketal 2.
2. What ultimate product would you expect to be obtained if the ethyl acetoacetate was not protected as the ethylene ketal prior to reaction with phenylmagnesium bromide?
3. What is the purpose of the "tiny crystal of iodine" that you added to the Grignard reaction mixture?
| Go to: | Instructions for Printing this Document Chem3D03 Lab Manual. |
30dec96; wjl