Dibenzoylmethane is synthesized by base-catalyzed condensation of benzaldehyde with acetophenone followed by halogenation, elimination and hydrolysis:
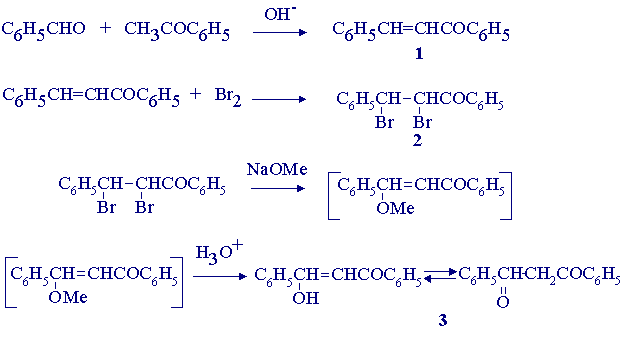
The enol content of the isolated ketone is determined by rapid bromination of the enol, destruction of excess Br2and determination of the bromoketone by an iodometric titration:

The first part of the preparation should be carried out during the preceding laboratory period. Start experiment #5b at the beginning of this period.
(i) Synthesis of Benzalacetophenone (1)
Pure acetophenone (6.5 g) is added to a solution of sodium hydroxide (2.75 g) in water (24.5 mL) and 95% ethanol (15 mL). the mixture is vigorously stirred while one equivalent of benzaldehyde is added with the temperature maintained between 20o and 30o. After 2 to 3 hours the mixture should become so thick that stirring is no longer effective. Stand in refrigerator overnight, filter and wash with water until washings are neutral to litmus. Finally, wash with 5 mL of ice cold ethanol. Air-dry the product (you should expect 10-11 g) and record its yield and melting range.
(ii) Synthesis of Dibenzoylmethane (3)
The crude benzalacetophenone (10 g) is dissolved in carbon tetrachloride (15 mL), the solution is cooled in ice, and bromine (one equiv.) is added with stirring. The dibromide (2) is removed by filtration and washed with two 6 mL portions of ethanol. The yield of crude dibromide should be ~15.5 g.
The dibromide (7.5 g) is added to methanol (7.5 mL) and a solution of sodium (1 g) in methanol (7.5 mL)* is added rapidly with stirring. The mixture is boiled under reflux for one hour. Sufficient 10% hydrochloric acid (3.5 mL) is added to neutralize the mixture; then a further 2 mL is added. After boiling this mixture for 5 minutes, water (7.5 mL) is added and the flask is cooled in ice water. The crude dibenzoylmethane (3) is collected, washed with 2.5 mL of cold 50% methanol and then with water until free of acid. Recrystallization of the air-dried, crude product from methanol should yield about 3 g of dibenzoylmethane. Record the melting point of the product. Dibenzoylmethane exists in three polymorphic forms melting at 73o, 78o and 81o.
(iii) Determination of Concentration of Enolic Form in Methanolic Solution
A 50 mL portion of a 0.04 M methanolic solution* of dibenzoylmethane is placed in a flask and maintained at 25oC for one hour. A 0.1 M methanolic solution (50 mL) of bromine is added, the flask is shaken, and 10 mL of a colourless methanolic solution of b-naphthol** is added within 20 seconds. The solution is stirred rapidly to dissolve the naphthol quickly. An aqueous solution of potassium iodide (50 mL, approx. 0.1M) is added and the liberated I2 is titrated against standard 0.1 M aqueous sodium thiosulfate to the colourless end point.
Calculate the keto-enol equilibrium constant for 3 in methanol.
* Consult demonstrators before preparing this.
Questions
What assumption(s), if any, is (are) involved in estimating the equilibrium constant from your data?
How could you remove the uncertainty introduced by these assumptions if you had more laboratory time?
Consider alternative methods for measuring the equilibrium constant, as well as employing the present method in a more time-consuming way.
Write detailed mechanisms for each stage of the preparation, and for the chemistry
involved in the enol-content determination.
(iv) Synthesis of trans-Cinnamic Acid
The condensations of active methylene compounds with aldehydes and ketones are known as Knoevenagel condensations. They are catalyzed by weak bases such as amines or carboxylate anions. Knoevenagel condensations involving malonic acid as the active methylene component usually involve spontaneous decarboxylation. For this reason they are sometimes called Doebner condensations or Doebner modifications of the Knoevenagel condensation.
![]()
In a round-bottomed flask fitted with a reflux condenser are placed 0.10 mole of freshly distilled benzaldehyde, 0.11 mole of malonic acid, 25 mL of 95% ethanol, and 2.5 mL pyridine. The mixture is heated under gentle reflux for 6 - 8 hours (during two lab periods). When the mixture has cooled, the large mass of crystals is broken up with a spatula and the reaction mixture is cooled in an ice bath. The solid is collected on a Buchner funnel and washed with 2 X 3-mL of cold 95% ethanol. The crude cinnamic acid is recrystallized from ethanol and the crystals are air-dried after filtration. The product is a white solid, 11-12.6 g (75 - 85%). Record the yield, m.p., and ir spectrum of your product.
QUESTIONS
1. Write mechanisms for each of the steps involved in the synthesis of dibenzoylmethane.
2. Write the mechanism for the Knoevenagel condensation. What is the probable mechanism by which the double bond in the final product (cinnamic acid) arises?
3. Why is the product trans- and not cis-cinnamic acid? If you can think of two possible reasons, suggest an experiment that could help you to decide which reason is correct.
__________________________________________________________________________
* Prepare this solution as accurately as you can. Make sure you use your volumetric
flask.
** 0.8 g in 10 mL
| Go to: | Instructions for Printing this Document Chem3D03 Lab Manual. |
30dec96; wjl