The Robinson annulation is a tremendously useful procedure for the synthesis of six-membered ring compounds, particularly in the steroid field. It consists of a Michael addition to yield an intermediate which undergoes concomitant intramolecular aldol condensation under the reaction conditions. An example is the synthesis of D1,9-2-octalone (Eqn. 1); numerous other examples have been discussed in class.

In this experiment, the synthesis of 5,5-dimethylcyclohexane-1,3-dione (trivial name: dimedone) will be carried out. The synthesis is actually a modification of the normal Robinson annulation, in that initial Michael addition of diethyl malonate with mesityl oxide (4-methylpent-3-en-2-one) is followed by an intramolecular Claisen condensation rather than aldol condensation. The annulation product is hydrolysed and decarboxylated in situ to yield the cyclic 1,3-diketone (Eqn.2).
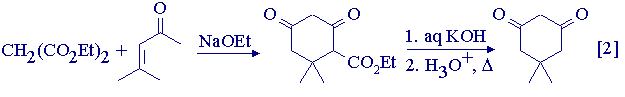
Mesityl oxide is a lachrymator, so the reaction (at least up to the decarboxylation stage) must be carried out in the fumehood.
(i) Annulation and hydrolysis of initial product.
In the fumehood, set up a 2- or 3-neck roundbottom flask fitted with a magnetic stirbar, Claisen adapter, reflux condenser with CaCl2 drying tube, and an addition funnel, and flame-dry it (make sure there are no volatile solvents in the vicinity!). While the apparatus is cooling, place sodium (1.15 g) in a 100-mL beaker under ca. 50-mL petroleum ether and cut it up carefully with a sharp blade and tweezers to pieces the size of a small pea (wear gloves). When the apparatus is cool to the touch, place absolute ethanol (30-mL) in the flask, and then add the sodium piece by piece through the top of the condenser with gentle stirring, at such a rate that the mixture boils. Replace the guard tube after each addition. Ensure that no more than two pieces of sodium accumulate in the flask at one time, and verify that no pieces become lodged in the condenser (if this happens, carefully push the sodium into the flask with a glass rod). During this procedure, measure diethyl malonate (0.05 mol) and mesityl oxide (0.05 mol) into separate 25-mL Erlenmeyer flasks. While the last bit of sodium is dissolving, place the diethyl malonate in the addition funnel and 5-mL of absolute ethanol in the (now empty) Erlenmeyer flask. Once the sodium has dissolved, add the malonate to the sodium ethoxide solution over 5 minutes. Add the 5-mL absolute ethanol to the addition funnel and add it all at once to the reaction mixture. Add the mesityl oxide followed by 5-mL absolute ethanol in exactly the same fashion, and then gently reflux the reaction mixture with stirring for 45 minutes.
Prepare 25-mL of ~4.5 M aqueous potassium hydroxide solution, and add it to the reaction mixture through the addition funnel. Continue refluxing for a further 45 minutes. Allow the mixture to cool,* remove the adapter, reflux condenser, and addition funnel, and arrange the apparatus for distillation. Distil off ca. 35-mL of the ethanol-water mixture, cool the residue in ice, and extract with 25-mL of diethyl ether, retaining the aqueous layer.
(ii) Decarboxylation
Return the aqueous layer from the previous step to the 100-mL roundbottom flask, acidify it to pH 1 with concentrated hydrochloric acid (do this in the hood), and reflux for 15 minutes. Cool the mixture in an ice bath and allow the product to crystallize. Set up a vacuum filtration apparatus and filter the crude product. Wash it with 25-mL water and 25-mL 35-60 petroleum ether, drying the product with suction after each washing. After recording the yield of the crude material, recrystallize it from aqueous acetone. Obtain the yield and melting point of the recrystallized material. Record the UV spectrum in ethanol, before and after addition of one drop of aqueous sodium hydroxide.
QUESTIONS
1. Write mechanisms for the formation of dimedone, as shown in Eqn. 2.
2. Note the absorption maxima in the UV spectrum of the product before and after the addition of base and explain the observed changes. Suggest another class of compound which behaves similarly.
3. Assign the peaks in the NMR spectrum of dimedone, and estimate the relative amounts of the diketone and any other species that may be present.
* may be left at this stage
| Go to: | Instructions for Printing this Document Chem3D03 Lab Manual. |
30dec96; wjl