
McMaster University - Chem3D03 Lab Manual
Ref.: McMurry, chapt.23
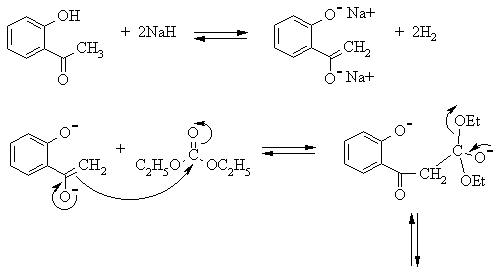
One of the important enolate condensation reactions is the Claisen condensation, which involves reaction of an enolate with the carbonyl compound of an ester. The enolate is generated by removal of a slightly acidic hydrogen from the a-carbon of a ketone, nitrile or ester using a relatively strong base. The enolate then attacks the carbonyl carbon of an ester to yield an intermediate which undergoes rapid loss of the alkoxide leaving group to yield a b-dicarbonyl compound. As usual, all steps in the sequence are completely reversible; the reaction is driven to the product side by deprotonation of the relatively acidic product by the alkoxide ion produced in the condensation. The final product is therefore a new enolate, which can be extracted from the reaction mixture by washing with water, in which it is soluble because it is a salt. It is finally liberated by acidifying the aqueous extract.
The synthesis of 4-hydroxycoumarin involves reaction of o-hydroxyacetophenone, which has two nucleophilic sites (the methyl carbon and the phenol oxygen), with diethyl carbonate, which has two leaving groups attached to its carbonyl carbon. Because of the presence of the second nucleophile and leaving group, the initial Claisen condensation product undergoes an intramolecular transesterification reaction to yield the cyclic product:


This experiment will be done on milligram scale, using microscale equipment and techniques. Before coming to the lab, be sure to read the sections on distillations, extractions, and recrystallizations in the microscale document.
Microscale Preparation of 4-Hydroxycoumarin
Note: It is very important that all equipment used in this reaction be thoroughly dried in an oven at 110 oC for 30 minutes just prior to use. Upon removal from the drying oven, it should be allowed to cool to room temperature in a desiccator.
Weigh and place 85 mg (2.13 mmol) of sodium hydride (60% dispersion in mineral oil) in a 10-mL roundbottom vial containing a magnetic stirring bar. Now add 3.0 mL of dry toluene. To the flask attach a Hickman still fitted (at the top) with an air condenser protected (again, at the top) by a calcium chloride drying tube.
CAUTION: Sodium hydride (NaH) is a flammable solid which reacts violently with water to produce hydrogen gas. Dispense in the hood. Toluene is distilled and stored over molecular sieves. Dispense this solvent in the hood as well.
In rapid order, place 133 ml (150 mg, 1.1 mmol) of o-hydroxyacetophenone, 3.0 mL of dry toluene, and 333 ml (324 mg, 2.75 mmol) of diethyl carbonate in a stoppered 10-mL Erlenmeyer flask, using dried syringes to dispense the liquids. Remove the air condenser from the distilling head and add this solution, with stirring, to the reaction flask as rapidly as possible using a Pasteur pipet. The resulting solution turns yellow. Immediately reattach the air condenser.
Place the reassembled apparatus in a sand bath and rapidly raise the temperature of the bath to about 175 oC.
Collect the ethanol and toluene distillate (1.0 mL in 2 or 3 fractions) in the collar of the Hickman still, and then remove the apparatus from the heat source. Allow the reaction solution to cool to room temperature, and then add 3.0 mL of water with stirring.
Transfer the resulting two-phase solution to a 15 mL centrifuge tube using a Pasteur pipet. Rinse the reaction flask with an additional 2 mL of water and add this to the centrifuge tube. Separate the toluene layer using a Pasteur filter pipet and transfer it to a second 15-mL centrifuge tube. Extract the toluene phase with 3 mL of water, and add the aqueous extract to the original water phase. This aqueous solution contains the water-soluble sodium enolate of 4-hydroxycoumarin. Cool the combined aqueous layers in an ice bath and acidify them by dropwise addition of concentrated HCl using a Pasteur pipet. The solid product should precipitate from the acidic aqueous phase. Add acid until the yellow color of the solution disappears (~10 drops). Collect the product by vacuum filtration using a Hirsch funnel.
Recrystallize the crude 4-hydroxycoumarin from 50% aqueous ethanol using a Craig tube. Dry the material on a piece of filter paper. Weigh the dried product and calculate the percent yield. Determine the melting point and obtain an IR spectrum in a KBr pellet.
| Go to: | Instructions for Printing this Document Chem3D03 Lab Manual. |
3mar98; wjl