McMaster University - Chem3D03 Lab Manual
References McMurry, 14.8,14.9, 31.7 Ege, 17.4, 28 Carey & Sundberg, Chapt.7 Fieser and Williamson, Organic Experiments, 5th Ed., p. 184)
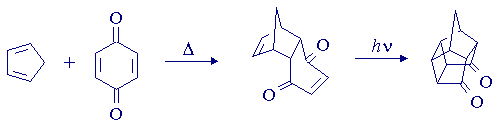
This experiment demonstrates the remarkable synthetic utility of cycloaddition reactions, which are important because they involve the formation of two C-C bonds in a single chemical step, and usually proceed with a high degree of (predictable) stereospecificity and regiospecificity. The first step in the synthesis of pentacyclo[5.4.0.02,6.03,10.05,9] undecane-8,11-dione, a pentacyclic cage compound, is a Diels-Alder reaction, which is an example of a thermal [4+2]-cycloaddition. The reaction involves the addition of a 4
p-component (the diene) to a 2p-component (the dienophile) in a 6p-electron process, which is "allowed" under thermal conditions by the rules of orbital symmetry. The second step is an intramolecular [2+2]-cycloaddition, which occurs upon irradiation of the initial product with UV light. The [2+2]-cycloaddition, involving addition of one 2p-component to another, is "thermally forbidden" but "photochemically allowed", suggesting that it will proceed only when one of the components is in an electronic excited state. Thermal reactions almost always occur in the electronic ground states of molecules. UV irradiation is the most important means of promoting molecules to electronic excited states, which have much different electron distributions than molecules in the electronic ground state and hence generally show much different reactivity.
Cyclopentadiene is a very reactive molecule which dimerizes if kept at room temperature for very long (another [4+2]-cycloaddition reaction!). The dimerization can be reversed by heating dicyclopentadiene, the Diels-Alder dimer of cyclopentadiene, to its boiling point with removal of the monomer as it is formed by distillation. To retard the reversion of cyclopentadiene to its dimer, keep it at 0oC and use it as quickly as possible after you make it.
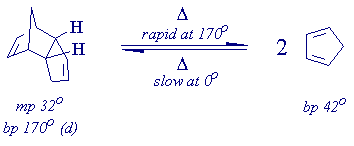
(i) Thermal Cracking of Dicyclopentadiene (Work in groups of 4 in the fume hood)
Decompose dicyclopentadiene (70 mL) by heating it in a 100 mL roundbottom flask fitted with a fractionating column, a condenser, and a receiver cooled with ice. The fractionating column can be made by inserting a spiral of steel wool into the glass column provided in your locker. When you have collected 20-25 mL of cyclopentadiene (~60 min distillation), dry it, if it is cloudy, with a few chunks of anhydrous calcium chloride, and divide it up into four 5 mL portions. Use it immediately.
[Important. Real organic chemists find the characteristic smell of cyclopentadiene and other olefinic or polyolefinic compounds to be positively enticing. However, as with all chemicals, it is important to take care to minimise prolonged exposure, as it may induce a headache. Work cleanly, dispose of waste, and wash ALL the apparatus that you have used for the cyclopentadiene preparation IN THE FUMEHOOD.]
(ii) Preparation of the Diels-Alder adduct, 4a,8a-dihydro-5,8-methano-1,4-naphthoquinone
Place 0.062 mol of pure (i.e. colourless - if it's not colourless, it needs to be sublimed; see your demonstrator) 1,4-benzoquinone in a 250-mL roundbottom flask, add 100 mL 95% ethanol and a magnetic stirbar, and place the flask in an ice bath. Stir the suspension magnetically, and add 0.08 mol of freshly distilled cyclopentadiene over a period of about 45 seconds. Stopper the flask and continue the stirring for a further 30 minutes. Transfer the flask to the rotary evaporator, and distill off the ethanol. Recrystallize the residue from ca. 120-180 mL of light petroleum spirits. Record the yield, melting point, and IR spectrum (in CCl4).
(iii) Preparation of pentacyclo[5.4.0.02,6.03,10.05,9]undecane-8,11-dione
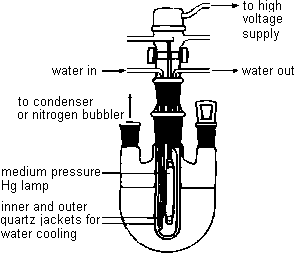 Dissolve 1.0 g of the Diels-Alder adduct in 50-mL of ethyl acetate in
a 100-mL Erlenmeyer flask. Label the flask and give it to your demonstrator, as it will be
irradiated during the following week with a 450-watt medium pressure mercury lamp. After
every 30 minute interval, your demonstrator will remove an aliquot of the solution for you
to analyse by TLC.
Dissolve 1.0 g of the Diels-Alder adduct in 50-mL of ethyl acetate in
a 100-mL Erlenmeyer flask. Label the flask and give it to your demonstrator, as it will be
irradiated during the following week with a 450-watt medium pressure mercury lamp. After
every 30 minute interval, your demonstrator will remove an aliquot of the solution for you
to analyse by TLC.
The irradiated solution will be returned to you the following week. Transfer it to a roundbottom flask, and evaporate the solvent on the rotary evaporator.
(iv) Purification by Chromatography
One of the more difficult tasks in synthetic organic chemistry is the purification of products. It is one of the more important problems however, particularly in the pharmaceutical industry where only extremely high purity compounds may be sold (would you eat anything you've made in the lab?).
Historically, there are two approaches used for product purification: distillation, where separation is based on differential vapour pressure, and recrystallization, which relies on selective solubility and crystal growth. While both are extremely useful techniques, especially for large scale preparations, the separation of closely related materials or small quantities of material is extremely difficult.
To solve such problems, a series of separation techniques - chromatography - have been developed. In these cases, the solute (compound to be purified) is dissolved in an inert medium (gas or liquid - the mobile phase) and is passed over (eluted) an immobile or stationary phase. Separation occurs because the solute reversibly adsorbs on the stationary phase. The partition between solvent and stationary phase results in the separation. Something which does not absorb well on the stationary phase will elute (move along the immobile phase) more rapidly than something which adsorbs well.
Many types of chromatography exist: e.g., gas chromatography (gaseous mobile phase, liquid / solid immobile phase), and liquid chromatography (organic solvent mobile phase, inorganic oxide - e.g. silica (SiO2) or alumina (Al2O3) - stationary phase).
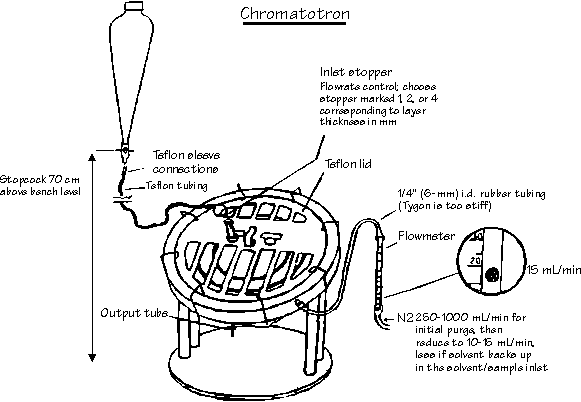
In this part of the experiment, you will purify your pentacyclic cycloadduct using thin layer chromatography and a Chromatotron. The latter is a "preparative layer chromatography" instrument: separation is accelerated by spinning the plate.
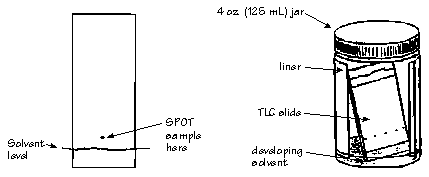
You will use thin layer chromatography (TLC) to select a suitable solvent for the separation. A TLC plate has a thin coating of silica gel (SiO2) or aluminum oxide (Al2O3) on glass, plastic or aluminum. To run a sample, "spot" (with a capillary - see Note 1) a dilute solution of your cycloadduct in ether, about 1 cm from the bottom of plate on 3 separate plates.
BE CAREFUL NOT TO TOUCH THE SURFACE OF THE PLATE WITH YOUR HANDS
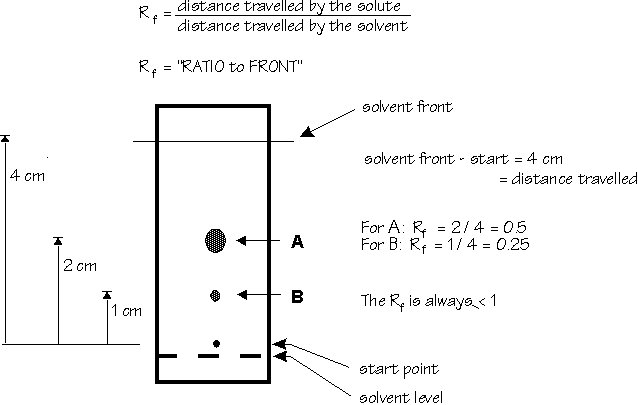
A solvent must now be chosen such that the major/desired component has an Rf = 0.3 [see note 2]. Run TLC's of your cycloadduct (separately) in hexane, dichloromethane and ethyl acetate. To do so, place the TLC plate (use tweezers) in a TLC developing chamber. The spot should be just above the solvent level (see Diagram below). Allow the solvent to elute almost to the top of the plate. Remove the TLC plate, and mark the solvent front (furthest distance the solvent has travelled) with a pencil. Calculate the Rf of the cycloadduct for each of the 3 solvent systems (see Note 2). (Knowing that SiO2 is polar, which solvent causes the cycloadduct to have the highest Rf - why?)
The Chromatotron is a thick layer plate chromatography instrument. The separation is accelerated by spinning the plate - isolation is facilitated by having the sample come off the plate in drops, which are then collected in fractions. Work in groups of FIVE for this part of the experiment.
Weigh accurately ca. 100mg of the cycloadduct and combine it with similar samples from the others in your group. Dissolve the ~0.5-g sample in a minimum of the appropriate solvent. Start the motor on the Chromatotron. Fill the plastic "pear shaped" reservoir with the appropriate solvent (see Diagram above). If the plate is dry, allow the solvent to flow rapidly (i.e., in steady stream) onto the plate until the solvent starts to emerge from the outlet tube. Stop the solvent flow. Drip the cycloadduct sample in the centre. With 1 or 2 mLs of solvent, wash all of the sample onto the plate. Reopen the solvent reservoir and allow it to drip at a rate of about 5 drops/second. Collect all fractions. In particular, collect fractions before, during and after the band containing the cycloadduct leaves the plate. Divide each of the three fractions into five equal portions, and redistribute them.
Spot these 3 fractions on TLC, elute, and see which of them are pure. Remove the solvent from each fraction in a preweighed flask on the rotovap. Weigh the flask and calculate the % recovery. Take the melting point of both your purified and crude samples. The literature melting point can be found in: A.P. Marchand and R.W. Allen, J. Org. Chem. 34, 1596 (1974).
1) Look at your TLC plates under the UV lamp. How many spots are there? What are they?
2) Did the separation purify the product? How do you know?
3) In which solvent system did the cycloadduct move highest in the plate (highest Rf)? Why?
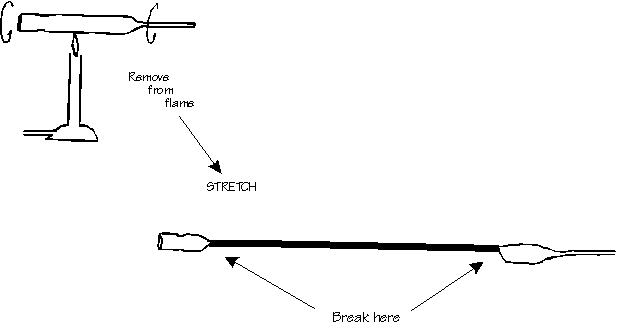
Note 1. Drawing capillaries
Light a bunsen burner AFTER MAKING SURE THERE ARE NO FLAMMABLE SOLVENTS AROUND. Adjust the air valve so that the flame is blue, not yellow. Heat, while turning, a pasteur pipette until it is red hot. Remove it from the flame and stretch it smoothly and rapidly. Break off the thick ends. Small capillaries can be made in the same way from melting point tubes.
Note 2. Determination of Rf factors
1. If the Diels-Alder adduct of cyclopentadiene and 1,4-benzoquinone is heated at 100oC in toluene solution for 24 hours, one obtains an isomer which exhibits similar IR and 1H nmr spectra to those of the initial adduct. What is the structure of the isomer? Would you expect it to undergo intramolecular photochemical [2+2] cycloaddition like the original adduct? Why?
2. Predict the product of the following reaction:
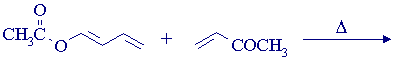
3. Write mechanisms to explain the following reaction, and identify A and B. There are six other possible (isomeric) products of the first step in the sequence. Identify them and explain why they're not formed.
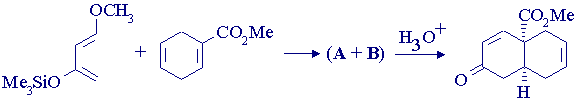
| Go to: | Instructions for Printing this Document Chem3D03 Lab Manual. |
13mar98; wjl