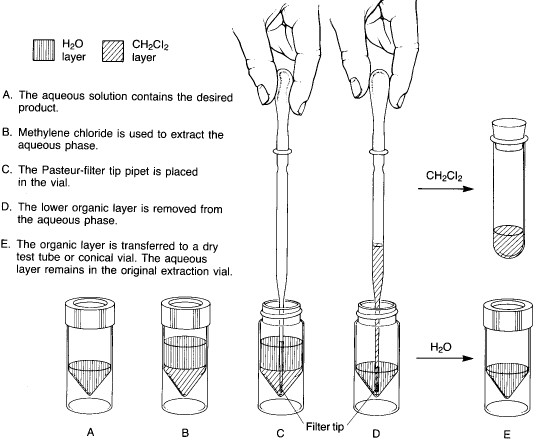
Method A - solvent heavier than water
| McMaster University - Chem3D03 Lab Manual | 1997/98 |
Microscale Laboratory Techniques - Extractions
In microscale experiments, the conical reaction vial is the glassware item used for extractions. The two immiscible liquid layers are placed in the vial, and the top is sealed with a cap and a Teflon insert (with the Teflon side toward the inside of the vial). The vial is shaken to provide thorough mixing between the two liquid phases. As the shaking continues, the vial is vented periodically by loosening the cap and then tightening it again. After about 5-10 seconds of shaking, the cap is loosened to vent the vial, retightened, and the vial is allowed to stand upright in a beaker until the two liquid layers separate completely.
Two basic procedures are possible, depending on whether the solvent being used to extract the desired product is heavier or lighter than water. Method A is employed for extractions where the lower layer is a heavy solvent such as dichloromethane:
Method A - solvent heavier than water |
|
Method B is employed for extraction with a solvent which is lighter than water, such as diethyl ether. Note that in this technique, one draws both phases into the pipet and then returns the heavy (aqueous) phase to the conical vial. Ether is so volatile that it is often difficult to hold it in the pipet. Use of a filter-tip pipet for this procedure will help prevent the volatile organic layer from squirting out in an uncontrolled way.
Method B - solvent lighter than water |
|
| Go to: | Instructions for
Printing this Document |
wjl; 25-nov-97