An Introduction to the Electronic Structure of Atoms
and Molecules
Dr. Richard F.W. Bader
Professor of Chemistry / McMaster University / Hamilton,
Ontario
|
The Electronic Basis of the Periodic Table
The hydrogen-like orbitals for a many-electron atom are listed in order
of increasing energy in Fig. 4-2. This energy level
diagram differs from the corresponding diagram for the hydrogen atom, a
one-electron system. In the many-electron atom all orbitals with the same
value of the principal quantum number n do not have the same energy
as they do in the case of hydrogen. For the many-electron atoms, the energy
of an orbital depends on both n and l, the energy increasing
as l increases even when n is constant. For example, from
Fig.
4-2 it is evident that the 3d orbital possesses a higher energy
than does the 3p orbital which in turn has a higher energy than
does the 3p orbital. The reason for this difference between the
one- and the many-electron case will be discussed below. The energy of
the orbital is still independent of the magnetic quantum number m.
Thus when l = 1, there are three
p orbitals which are still
degenerate (all possess the same energy) and this is indicated by the three
open circles which are superimposed on each of the p levels. The
open circles thus represent the number of available orbitals or the degeneracy
of each orbital energy level.
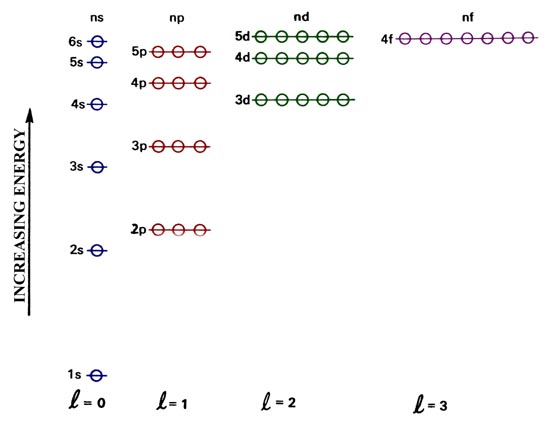
Fig. 4.2. An orbital energy level diagram for a
many-electron atom.
With the aid of this energy level scheme and
the Pauli principle we may proceed to build up the electronic structures
of all the atoms. We do this by asssigning electrons one at a time to the
vacant orbital which possesses the lowest energy. An orbital is "filled"
when it contains two electrons with their spins paired.
Hydrogen. The nuclear charge is 1 and the single electron
is placed in the 1s orbital. The electronic configuration is 1s1.
Helium. The nuclear charge is increased by one unit to
2 and the extra electron is again placed in the 1s orbital, with
its spin opposed to that of the electron already present. The electronic
configuration is 1s2.
Lithium. The nuclear charge is 3 and the third electron,
because of the Pauli principle, must be placed in the 2s orbital as the
1s orbital is doubly occupied. The electronic configuration of lithium
is therefore 1s22s1.
We can now answer the question as to why the 2s
orbital is more stable than the 2p orbital, i.e., why Li is described
as 1s22s1
and not as 1s22p1.
The two inner electrons of lithium (those in the 1s orbital) partially
shield the nuclear charge from the outer elctron. Recall that as n
increases, the average distance between the electron and the nucleus increases.
Thus most of tthe electron density of the electron with n = 2 will
lie outside of the charge density of the two inner electrons which have
n
= 1. When the outer electron is at large distances from the nucleus and
thus essentially outside of the inner shell of electron density it will
experience a force from only one of the three positive charges
on the lithium nucleus. However, as the outer electron does have a small
but finite probability of being close to the nucleus, it will penetrate
to some extent the tightly bound electron density of the two 1s
electrons. In doing so it will "see" much more of the total nuclear charge
and be more tightly bound. The closer the outer electron can get to the
nucleus, that is, the more it can penetrate the density distribution of
the inner shell electrons, the more tightly bound it will be.
An electron in an s orbital has a finite probability
of being found right at the nucleus. An electron in a p orbital
on the other hand has a node in its density distribution at the nucleus.
Thus an s electron penetrates the inner shell density more effectively
than does a p electron and is consequently more tightly bound to
the atom. In a hydrogen atom, there are no inner electrons and both a 2s
and 2p electron always experience the full nuclear charge and have
the same energy. The crux of this penetration effect on the energy is that
the inner shell electron density does possess a finite extension in space.
Thus an outer electron can penetrate inner shell density and the screening
effect is reduced. If the inner shell density was contracted right onto
the nucleus, then no matter how close the outer electron came to the lithium
nucleus, it would always experience only a charge of +1. This dependence
of the orbital energies on their l value is aptly called the penetration
effect.
The electron density of a d electron is concentrated
even further away from the nucleus than is that of a p electron.
Consequently, the orbital energy of a d electron is even less stable
than that of a p electron. In some atoms the penetration of the
inner shell density by a d electron is so slight that its energy
is raised even over that of the s electron with the next highest
n
value. For example, a 3d electron possesses a higher energy than
does a 4s electron in the atoms Sc to Zn with the exceptions of
Cr and Cu. The penetration effect in these elements overrides the principal
quantum number for d electrons in determining their relative energies.
Notice that the configuration 1s22s1
for lithium overcomes the difficulties of our earlier attempts to describe
the electronic structure of this atom. The Pauli principle, of which we
were ignorant in our previous attempt, forces the third electron to occupy
the 2s orbital and forces in turn the beginning of a new quantum
shell, that is, a new value of n. Thus lithium, like hydrogen, possesses
one outer electron in an s orbital. Since it is only the outer electron
density which in general is involved in a chemical change, lithium and
hydrogen should have some chemical properties in common, as indeed they
do. Hydrogen is the beginning of the first period (n = 1) and lithium
marks the beginning of the second period (n = 2).
Beryllium. The nuclear charge is 4 and the electronic configuration
is 1s22s2.
Boron. Z = 5 and the electron configuration is 1s22s22p1.
Carbon. Z = 6. The placing of the sixth electron
of carbon requires some comment. It will obviously go into a 2p
orbital. But in which of the three should it be placed? Should it be placed
in the 2p orbital which already possesses one electron, or should
it be placed in one of the vacant 2p orbitals? If it is placed in
the occupied 2p orbital its spin must be paired with that of the
electron already present and the result would be a nonmagnetic carbon atom.
If, however, it is placed in one of the vacant 2p orbitals it may
be assigned a spin parallel to the first electron. The question is decided
on the grounds of which situation gives the lowest energy. As a result
of the Pauli principle, two electrons with parallel spins (both up or both
down) have only a very small probability of being close to one another.
In fact the wave function which describes the two-electron case for parallel
spins vanishes when both electrons approach one another. When the wave
function vanishes, the corresponding probability distribution goes to zero.
On the average, then, electrons with parallel spins tend to keep out of
each other's way. Two electrons with paired spins, whether in the same
or different orbitals are not prevented by the Pauli principle from being
close to one another. The wave function for this situation is finite even
when they are on top of one another! Obviously, two electrons with parallel
spins will have a smaller value for the electrostatic energy of repulsion
between them than will two electrons with paired spins. This is a general
result which holds almost without exception in the orbital approximation.
It is known as one of Hund's rules as he was the first to state it. Thus
the most stable electronic configuration of the carbon atom is 1s22s22p2()
where
we have emphasized the fact that the two 2p electrons have parallel
spins and hence must be in different 2p orbitals.
Nitrogen. Z = 7. Because of Hund's rule the
electronic configuration is
1s22s22p3()
i.e., one electron in each of the 2p orbitals. The configuration
with the largest possible component of the spin magnetic moment will be
the most stable.
Oxygen. Z = 8. One of the 2p electrons must
now be paired with the added electron, but the other 2p electrons
will be left unpaired.
1s22s22p4()
(Only the number of unpaired electrons is indicated by the arrows.)
Fluorine. Z = 9. The configuration will be
1s22s22p5()
Neon. Z = 10. The tenth electron will occupy the
last remaining vacancy in the second quantum shell (the set of orbitals
with n = 2).
1s22s22p6
Thus neon represents the end of the second period and all the electrons
have paired spins.
When all the orbitals in a given shell are doubly
occupied, the resulting configuration is called a "closed shell." Helium
and neon are similar in that they both possess closed shell configurations.
Because neither of these elements possesses a vacancy in its outer shell
of orbitals both are endowed with similar chemical properties. When the
orbitals belonging to a given l value contain either one electron
each (are half-filled) or are completely filled, the resulting
density distribution is spherical in shape. Thus the
electron density distributions of nitrogen and neon, for example, will
be spherical.
Reference to Fig. 4-2 indicates
that the next element, sodium, will have its outer electron placed in the
3s orbital and it will be the first element in the third period.
Since its outer electronic structure is similar to that of the preceding
elements, lithium and hydrogen, it is placed beneath them in the periodic
table. It is obvious that in passing from sodium to argon, all of the preceding
outer electronic configurations found in the second period (n =
2) will be duplicated by the elements of the third period by filling the
3s and 3p orbitals. For example, the electronic structure
of phosphorus (Z = 15) will be
1s22s22p63s23p3()
and thus resemble nitrogen.
Argon. Z = 18. Argon will have filled 3s
and 3p orbitals and will represent the end of a period. Argon, like
helium and neon, possesses a closed shell structure and is placed beneath
these two elements in the periodic table.
The Transition Elements. The beginning of the fourth period
will be marked by the single and double occupation of the 4s orbital
to give potassium and calcium respectively. However, reference to the orbital
energy level diagram indicates that the 3d orbital is more stable
than the 4p orbital. Since there are five d orbitals they
may hold a total of ten electrons. Thus the ten elements beginning with
scandium (Z = 21) will possess electronic structures which differ
from any preceding them as they are the first elements to fill the d
orbitals. A typical electronic configuration of one of these elements is
that of manganese: [Ar]4s23d5.
The symbol [Ar] is an abbreviated way of indicating that the inner shells
of electrons on manganese possess the same orbital configuration as those
of argon. In addition, the symbol 3d5
indicates that there are five electrons in the 3d orbitals, no distinction
being made between the five different d orbitals. This series of
elements in which the 3d orbitals are filled is called the first
transition series. The element zinc with a configuration [Ar]4s23d10
marks the end of this series. The six elements from gallium to krypton
mark the filling of the 4p orbitals and the closing, with krypton,
of the fourth quantum shell and the fourth period of the table.
While the 3d orbitals are less stable than the 4s
orbitals in the neutral atom (with the exceptions of Cr and Cu) and are
filled only after the 4s orbitals are filled, the relative stability
of the 4s and 3d orbitals is reversed in the ionic forms
of the transition metals. For example, the configuration of the ion which
results when the manganese atom loses two electrons is Mn+2
[Ar]3d5 and not [Ar]4s23d3.
This is a general result. The d orbitals of quantum number n
are filled only after the s orbital of quantum number (n
+ 1) is filled in the neutral atom, but the nd orbital is more stable
than the (n + l)s orbital in the corresponding ion.
The fifth period begins with the filling of the 5s
orbital, followed by the filling of the 4d orbitals, which generates
the second transition series of elements. The period closes with the filling
of the 5p orbitals and ends with xenon.
The lanthanide and actinide elements. The sixth period
is started by the filling of the 6s orbital. The next element, lanthanum,
has the electronic configuration [Xe]6s25d1.
However, the next fourteen elements represent the beginning of another
new series as the filling of the 4f orbitals is now energetically
favoured over a continued increase in the population of the 5d orbitals.
Note that the very small penetration effect possessed by the 4f
orbitals (n = 4) delays their appearance until the sixth quantum
shell has been partially filled. There are fourteen elements in this series,
called the lanthanide series, since there are seven 4f
orbitals (l = 3 and 2 ´ 3+1 = 7).
The third transition series follows the lanthanide elements
as the occupation of the 5d orbitals is completed. This in turn
is followed by the filling of the 6p orbitals. The final period
begins with the filling of the 7s orbital and continues with the
filling of the 5f orbitals. This second series of elements with
electrons in f orbitals is called the actinide series.
The concept of atomic orbitals in conjunction with the
Pauli principle has indeed predicted a periodicity in the electronic
structures of the elements. The form of this periodicity duplicates
exactly that found in the periodic table of the elements in which the periodicity
is founded on the observed chemical and physical properties of the elements.
Our next task will be to determine whether or not our proposed electronic
structures will properly predict and explain the observed variations in
the chemical and physical properties of the elements.