Organophosphorous Chemistry
The Wittig reaction is one of the premier carbon-carbon bond forming reactions in organic chemistry. Traditionally, the reaction requires a strong base (ex. BuLi, LiHMDS), anhydrous conditions, and cryogenic temperatures. As well, triphenylphosphine derived phosphonium salts often give poor E/Z selectivity, and generate a stoichiometric amound of difficult to remove triphenylphosphine oxide. Our group has developed a robust methodology for performing Wittig reactions in aqueous conditions with bases as mild as sodium bicarbonate. The use of trialkylphosphine derived phosphonium salts affords very high E selectivity, and the resulting trialkylphosphine oxide is totally soluble in water. In many cases, the product is insoluble in the reaction media, allowing for facile isolation without the use of organic solvents in any stage of the reaction. We have also developed DualPhos, a useful reagent for the two-carbon homologation of aldehydes into alkenals in a two-step (one pot) process under aqueous of classical conditions. The product alkenals are valuable synthons, frequently encountered in pharmaceutical syntheses, total synthesis and organocatalysis. |
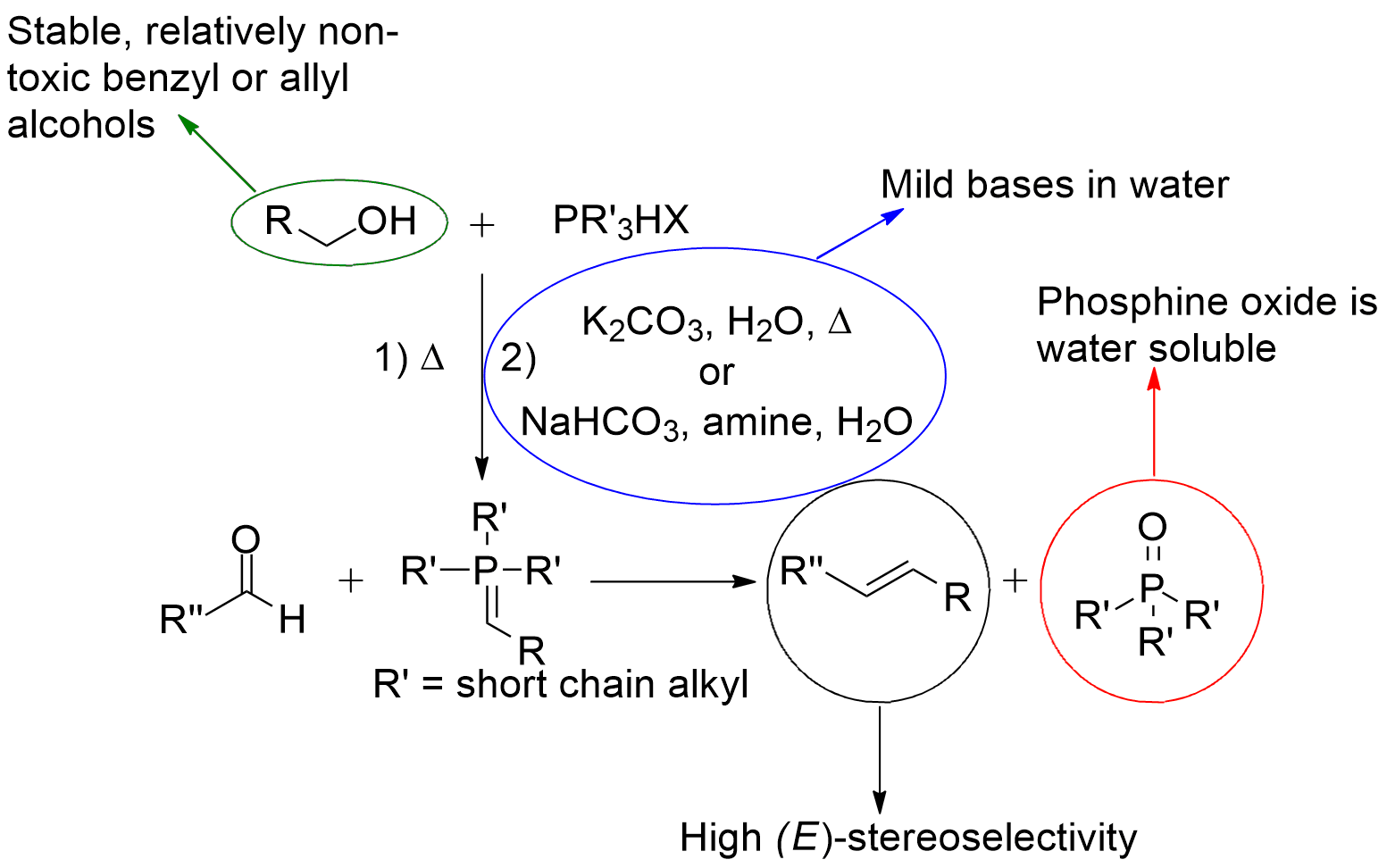 |
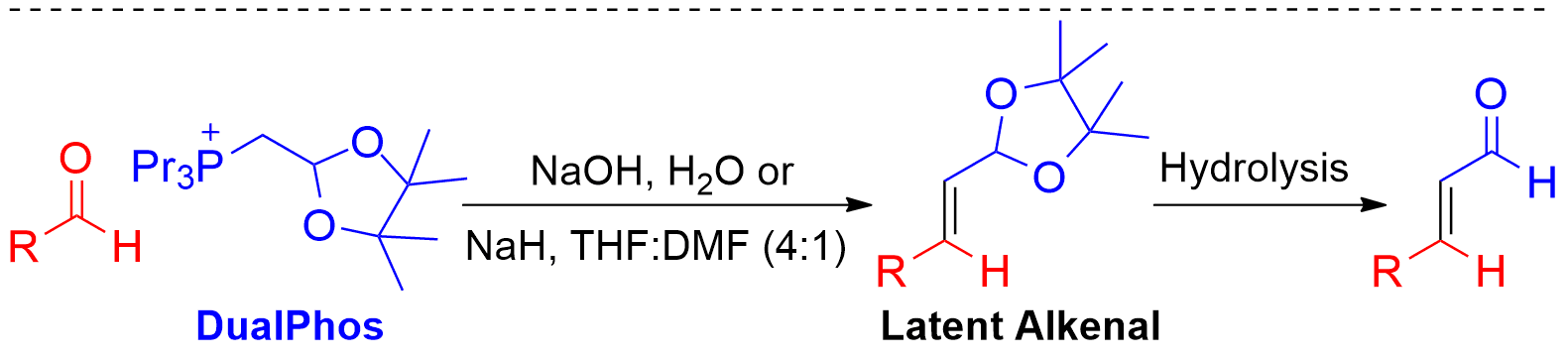 |
|
Notably, the latent alkenal intermediates generated by DualPhos are readily isolable and stable under a wide variety of conditions, including reductions (LAH and hydrogenations), oxidants, strong bases and organometallic compounds. They are also stable to a wide variety of Lewis acids, but are readily removed by FeCl3xH2O in acetone, 25% phosphoric acid, or Amberlite IR-120 acidic resin. Our group is actively pursusing the development of related reagents and expanding the scope of aqueous Wittig methodology. |
|
Relevant Articles DualPhos: a versatile, chemoselective reagent for two-carbon aldehyde to latent alkenal homologation application in the total synthesis of phomolide G. D. Mcleod, J. McNulty*, Roy. Soc. Open Sci. 3, 160374 (2016). Wittig Reactions of Trialkylphosphine-derived Ylides: New Directions and Applications in Organic Synthesis. J. McNulty*, D. McLeod, P. Das, C. Zepeda-Velázquez, Phosphorus. Sulfur, Silicon & Relat. Elements., 190, 619-632 (2015). Development of a robust reagent for the two-carbon homologation of aldehydes to (E)-α,β-unsaturated aldehydes in water. J. McNulty*, C. Zepeda, D. McLeod, Green Chemistry, 15, 3146-3149 (2013), A scalable process for the synthesis of (E)-pterostilbene involving aqueous Wittig olefination chemistry. J. McNulty*, D. McLeod, S., 54, 6303-6306 (2013). Mild chemical and biological synthesis of donor-acceptor flanked reporter stilbenes: Demonstration of a physiological Wittig olefination reaction. J. McNulty*, D. McLeod, Eur. J. Org. Chem. 6127-6131 (2012). Amine and sulfonamide promoted Wittig olefination reactions in water. J. McNulty*, D. Mcleod, Chem. Eur. J. 17, 8794-8798 (2011). An iterative approach toward the synthesis of discrete oligomeric p-phenylene vinylene organic dyes employing aqueous Wittig chemistry. J. McNulty*, D. McLeod, Tetrahedron Lett., 52, 5467-5470 (2011). A Direct Synthesis of Functionalized Styrenes and Terminal 1,3-dienes via Aqueous Wittig Chemistry with Formalin. P. Das, D. Mcleod, J. McNulty*, Tetrahedron Lett., 52, 199-201 (2011). Microwave-assisted aqueous Wittig reactions: Chemoselective, organic solvent and protecting group-free synthesis of functionalized alkenes. J. McNulty*, P. Das, D. McLeod., Chem. Eur. J. 16, 6756-6760 (2010). Highly stereoselective and general synthesis of trans-stilbenes and alkenes by means of an aqueous Wittig reaction. J. McNulty, P. Das, Eur. J. Org. Chem., 4031 (2009). |
|