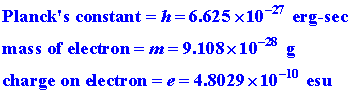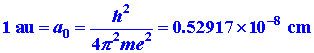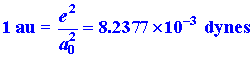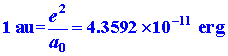An Introduction to the Electronic Structure of Atoms
and Molecules
Dr. Richard F.W. Bader
Professor of Chemistry / McMaster University / Hamilton,
Ontario
|
Units of Measurement used in Atomic Physics
The energies of electrons are commonly measured and
expressed in terms of a unit called an electron volt. An
electron volt (ev) is defined as the energy acquired by an electron when
it is accelerated through a potential difference of one volt.
Imagine an evacuated tube which contains two parallel separate
metal plates connected externally to a battery supplying a voltage V.
The cathode in this apparatus, the negatively-charged plate, is assumed
to be a photoelectric emitter. Photons from an external light source with
a frequency no
upon striking the cathode will supply the electrons with enough energy
to just free them from the surface of the cathode. Once free, the electrons
will be attracted by and accelerated towards the positively-charged anode.
The electrons, which initially have zero velocity at the cathode surface,
will be accelerated to some velocity u when
they reach the anode. Thus the electron acquires a kinetic energy equal
to ½ mu2
in falling through a potential of V volts. If the charge on the
electron is denoted by e this same energy change in ev is given
by the charge multiplied by the voltage V:
| (5) |
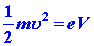
|
For a given velocity u in cm/sec, equation
(5)
provides a relationship between the energy unit in the cgs (centimetre,
gram, second) system, the erg, and the electron volt. This relationship
is:
The regular cgs system of units is inconvenient to use on
the atomic level as the sizes of the standard units in this system are
too large. Instead, a system of units called atomic units,
based on atomic values for energy, length, etc., is employed.
Atomic units are defined in terms of Planck's constant
and the mass and charge of the electron:
Length.
Force.
Force has the dimensions of charge squared divided by distance squared
or
Energy.
Energy is force acting through a distance or